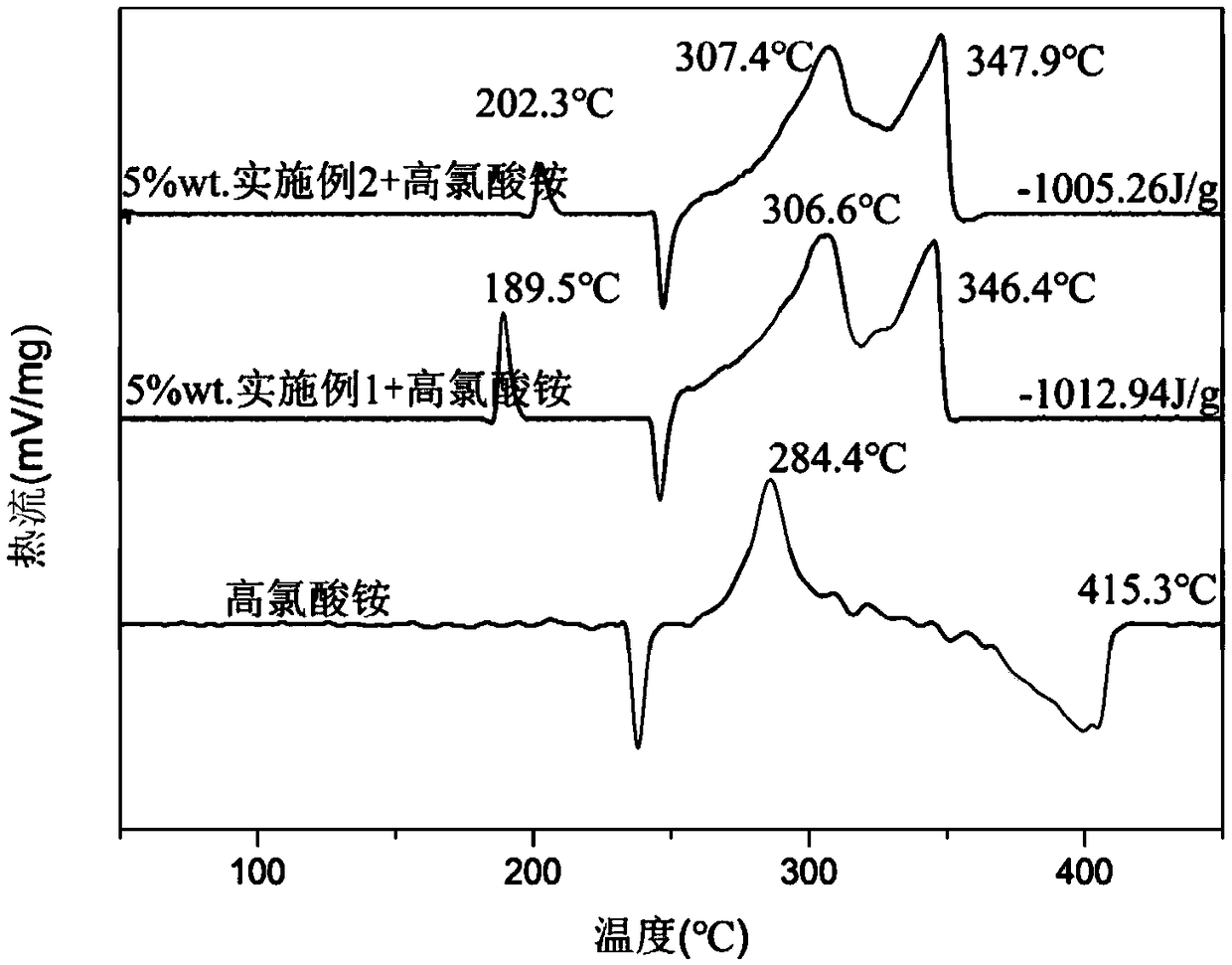
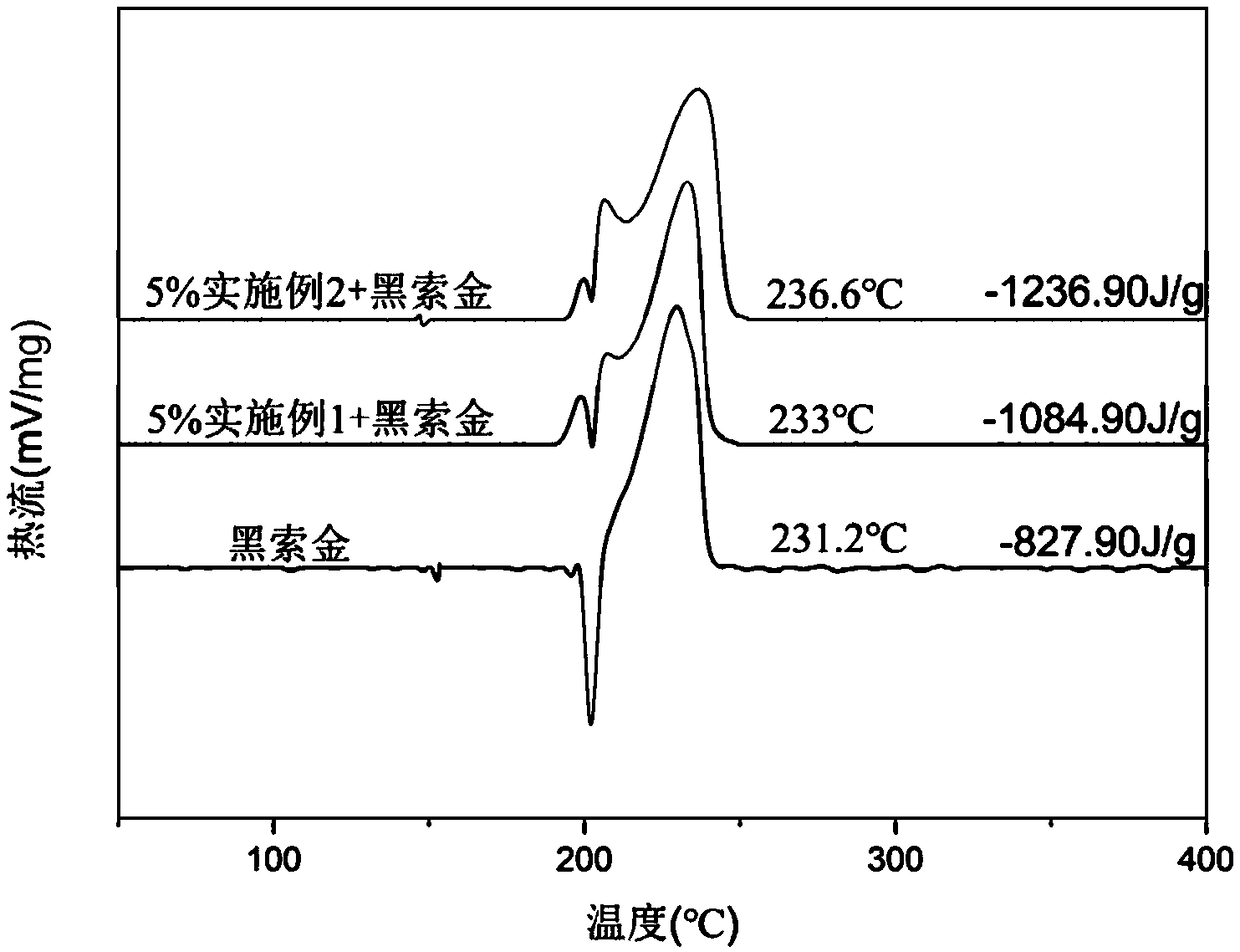
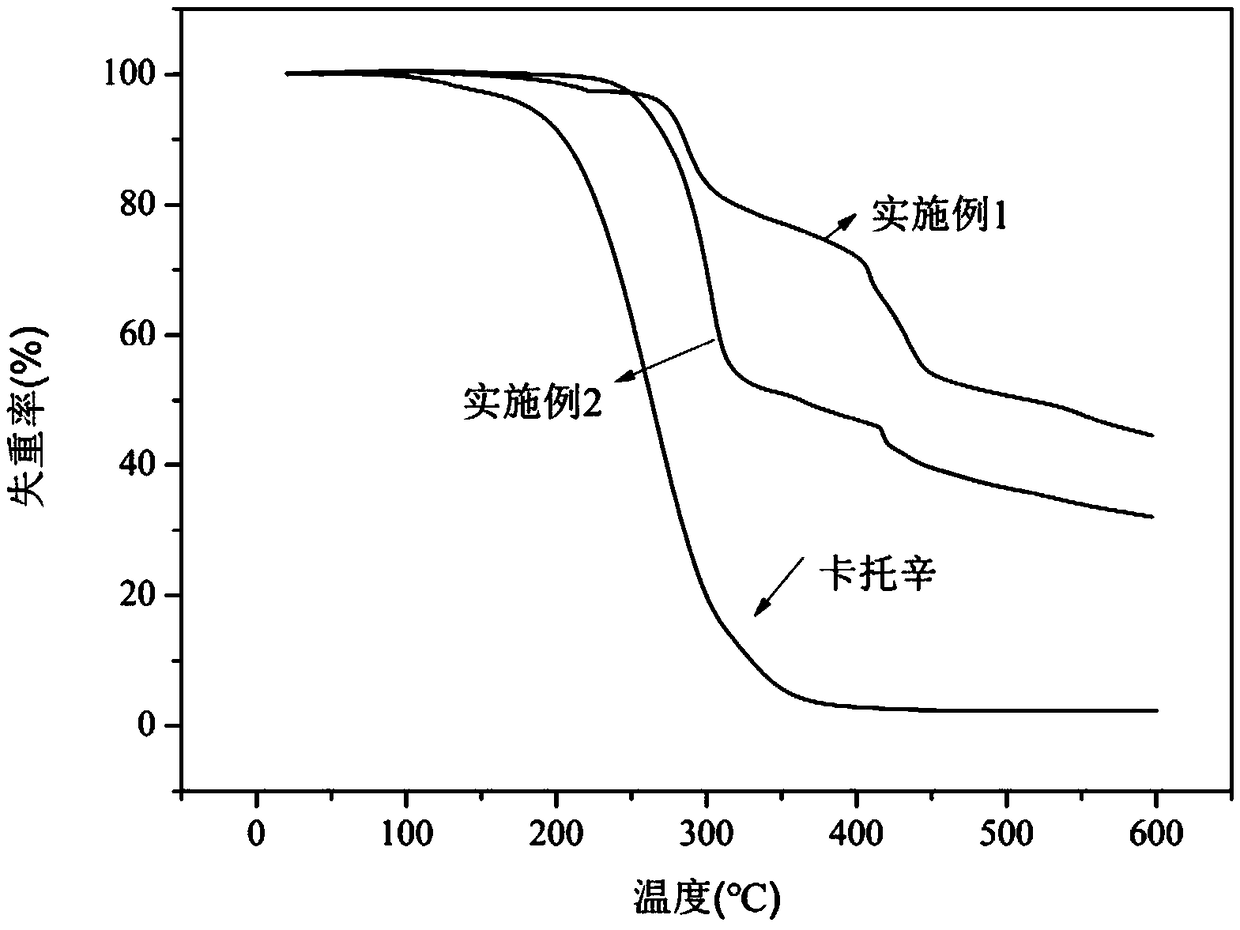
Benzoate-based burning rate catalyst containing dinuclear ferrocene group and preparation method of benzoate-based burning rate catalyst
A burning rate catalyst, ferrocene-based technology, applied in chemical instruments and methods, metallocene, organic chemistry, etc., can solve problems such as complex synthesis process, achieve simple operation, good thermal stability, and improve thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of bis-(ferrocenylmethyl-1,2,3-triazolylmethyl) terephthalate with the following structural formula
[0026]
[0027] Add 0.2230g (1mmol) di-2-ynylpropyl terephthalate and 0.4931g (2.05mmol) azidomethylferrocene to a 250mL round bottom flask, 2 Add 30mL of methanol under the atmosphere, stir evenly, then add dropwise 15mL of an aqueous solution containing 0.077g (0.3mmol) copper sulfate pentahydrate and 15mL of an aqueous solution containing 0.0604g (0.3mmol) of sodium ascorbate, stir at room temperature for 24h, and filter to obtain a crude product. The crude product was separated by column chromatography to obtain bis-(ferrocenylmethyl-1,2,3-triazolylmethyl) terephthalate, the yield was 82%, and the structural characterization data was: FT-IR (cm -1 ):3431m, 3155m, 2955w, 1715vs, 1626m, 1383m, 1335m, 1265vs, 1106vs, 1044m, 933m, 801s, 718m, 490s; 1 H NMR (400MHz, CDCl 3 ):δ8.04(s,4H),7.59(s,2H),5.43(s,4H),5.30(s,4H),4.29(s,4H),4.23(s,4H),4.18(vs, 10...
Embodiment 2
[0029] Preparation of bis-(ferrocenylmethyl-123-triazolylmethyl) phthalate of following structural formula
[0030]
[0031]In this embodiment, the di-2-ynylpropyl terephthalate in Example 1 is replaced with equimolar di-2-ynylpropyl phthalate, and the other steps are the same as in Example 1. Bis-(ferrocenylmethyl-1,2,3-triazolylmethyl) formate, the yield is 79%, and the structural characterization data is: FT-IR (cm -1 ):3427m, 3094m, 2955w, 1723vs, 1626w, 1445m, 1383m, 1272vs, 1120vs, 1058s, 940m, 822s, 753m, 635w, 490s; 1 H NMR (400MHz, CDCl 3 ): δ7.68(s,2H),7.62(s,2H),7.51(s,2H),5.30(s,8H),4.31(s,4H),4.19(s,14H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com