Synthesis method of indometacin and analogues thereof
A synthetic method, the technology of indomethacin, applied in the direction of drug combination, chemical instrument and method, organic compound/hydride/coordination complex catalyst, etc., can solve the limitations of functional groups and substrates, unfavorable indomethacin Molecular structure modification, biological function research, long steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
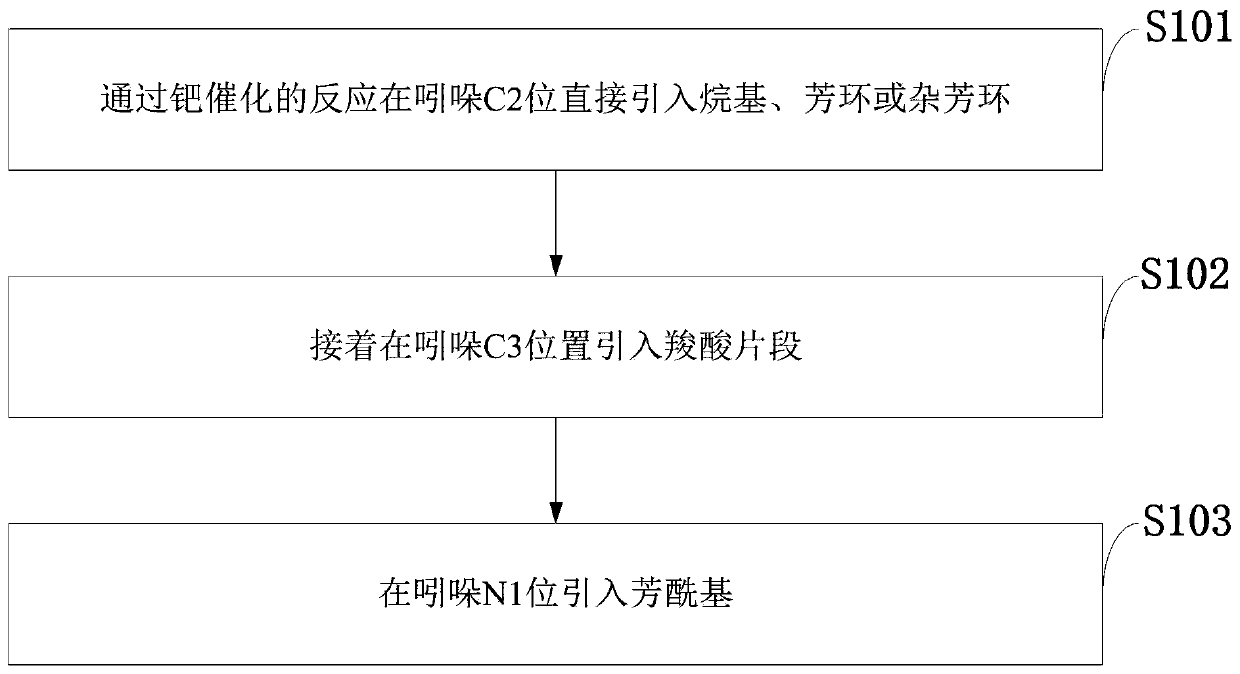
[0041] Such as figure 1 As shown, the synthetic method of indomethacin and its analogs provided by the embodiments of the present invention comprises the following steps:
[0042] S101: Directly introduce an alkyl group, an aromatic ring or a heteroaryl ring at the C2 position of indole through a palladium-catalyzed reaction;
[0043] S102: then introducing a carboxylic acid fragment at the indole C3 position;
[0044] S103: introducing an aroyl group at the N1 position of indole.
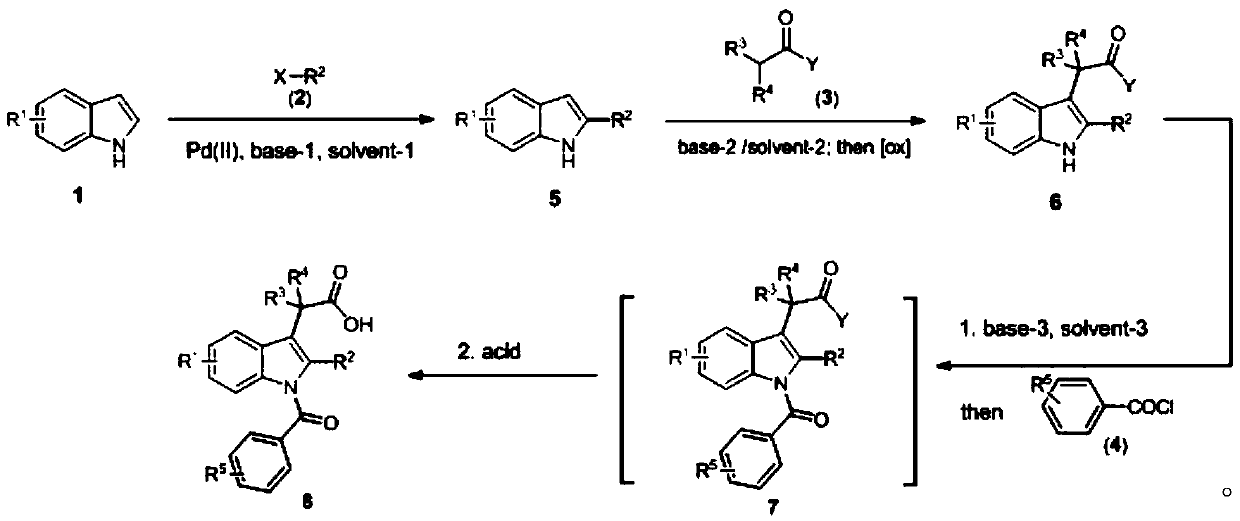
[0045] The synthesis method of indomethacin and its analogs provided in the examples of the present invention realizes the three-step synthesis method of indomethacin and its analogs starting from indole molecules (Formula 6).
[0046]
[0047] In a preferred embodiment of the present invention, R 1 , R 2 , R 3 , R 4 , R 5 Can be the same or different; R 1 Can be C4, C5, C6 and C7 alkyl, alkoxy, halogen, ester group, amide, amino, substituted aromatic ring and heteroaryl ring on indole 1...
Embodiment 1
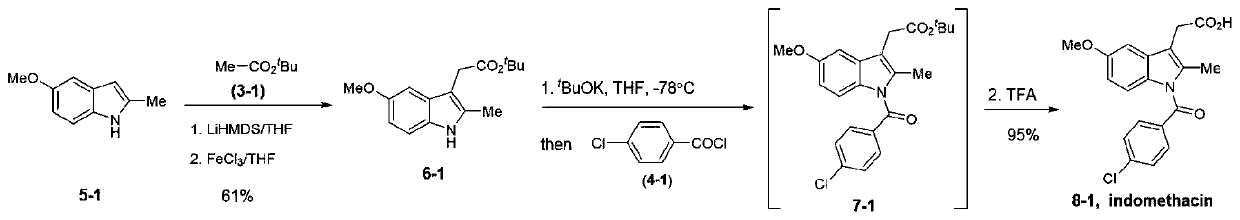
[0059] Embodiment 1: the synthesis of indomethacin (formula nine)
[0060]
[0061] At room temperature, compound 5-1 (161 mg, 1.0 mmol, 2.0 equiv.) and 3-1 (58 mg, 0.5 mmol, 2.0 equiv.) were dissolved in anhydrous tetrahydrofuran and cooled to -78 ° C, and then under nitrogen protection , LiHMDS (1.3M in THF, 1.54 mL, 2.0 mmol, 4.0 equiv.) was added dropwise. After the reaction solution was stirred at -78°C for half an hour, FeCl was added 3 (322 mg, 2.0 mmol, 4.0 equiv.). After reacting at -78°C for 2 hours, water was added to quench the reaction, then raised to room temperature and ethyl acetate was added, washed with water and saturated brine in turn, and dried over anhydrous sodium sulfate. The desired product 6-1 (84.3 mg, 61% yield) was obtained after filtration concentration column chromatography.
[0062] Add compound 6-1 (15mg, 0.054mmol, 1.0equiv.) to a 20mL reaction tube at one time, then add 4mL of anhydrous tetrahydrofuran under nitrogen protection, stir at...
Embodiment 2
[0071] Embodiment 2: the synthesis of indomethacin analogue 7-2 (formula ten)
[0072]
[0073] In a 100mL round bottom flask, add indole 1-1 (1.75g, 10mmol, 1.0equiv.), halogenated hydrocarbon 2-1 (xxmg, 10mmol, 1.0equiv.), Pd(OAc) 2 (224mg, 1.0mmol, 1.0equiv.), norbornene (norbornene, 1.88g, 20mmol, 2.0equiv.) and K 2 CO 3 (2.76g, 20mmol, 2.0 equiv.). Then add DMA (0.5M H 2 O), heated to 80 degrees Celsius and stirred for 18h. After the reaction is over, add 100mL of dichloromethane to dilute, filter to remove insoluble matter, wash the solution with water and saturated brine to remove DMA, and wash with Na 2 SO 4 dry. Filtration, removal of solvent, the crude product was purified by silica gel column chromatography to obtain target compound 5-2 (723mg, 28% yield)
[0074] The synthesis methods of compounds 6-2, 7-2 and 8-2 are the same as those of compounds 6-1, 6-2 and 7-2.
[0075]
[0076] Yellow solid, 732.3mg, 28% yield (10mmol scale)
[0077] 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com