Novel benzylideneacetone derivative and use thereof
A technology of benzyloxy and hydroxyl, applied in the field of new benzylidene acetone derivatives, which can solve the problems of limited use and high cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
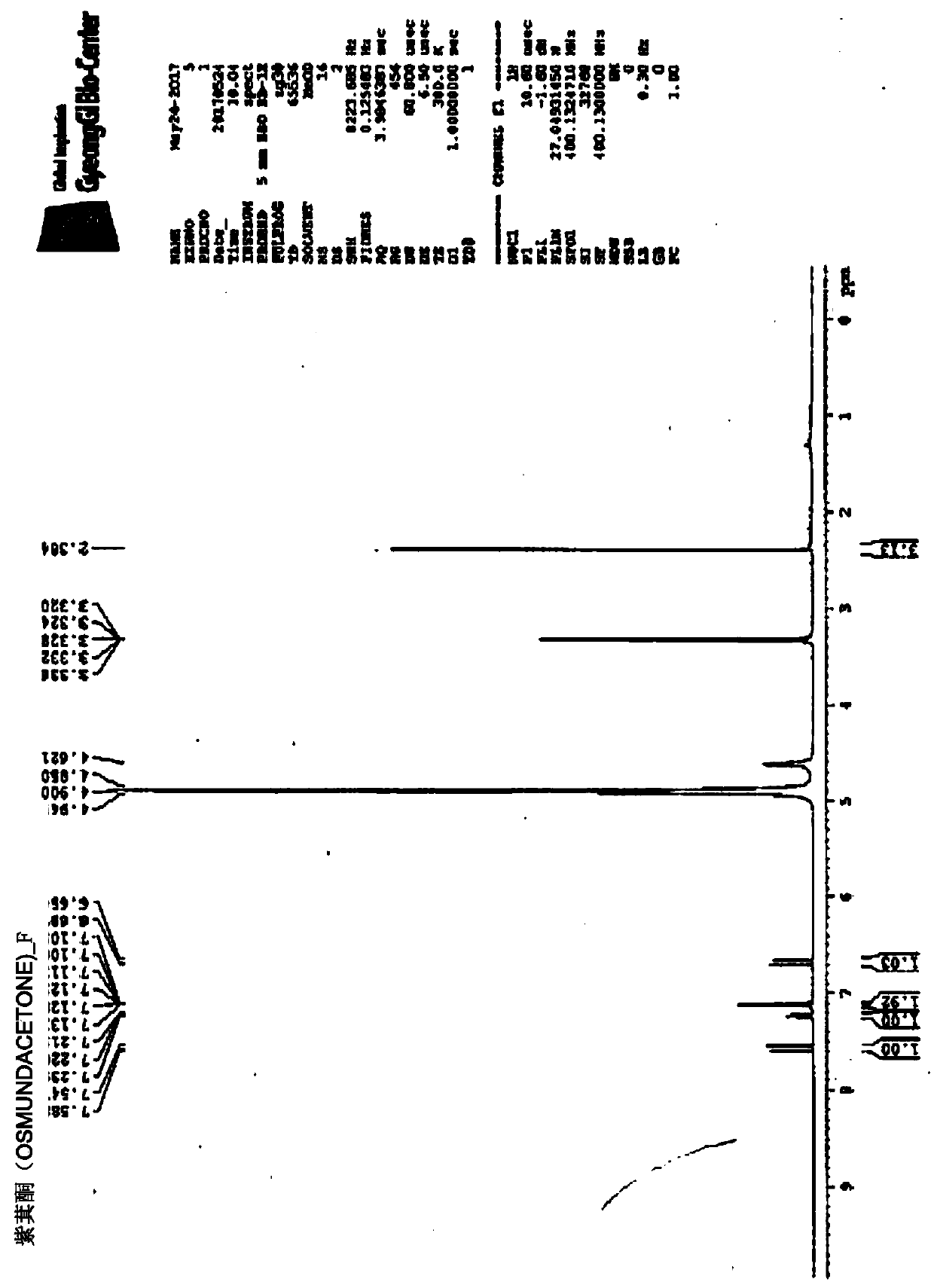
[0200] Synthesis of novel compounds
[0201] To identify substances with anticancer activity and osteoclast differentiation inhibitory activity, the compounds were isolated and purified from the synthetic mixture of each compound, and the chemical structure of each compound was determined by nuclear magnetic resonance (NMR) and mass spectrometry (MS).
[0202] The synthesis method of each compound and the specific NMR and MS analysis results are as follows:
Embodiment 1-1
[0204] Synthesis of (E)-4-(4-fluoro-3-hydroxyphenyl)but-3-en-2-one (KP3)
[0205] 【Table 1】
[0206]
[0207] Under cooling, boron tribromide (1M dichloromethane solution, 10ml) was added dropwise to 3-methoxy-4-fluorobenzaldehyde ((3-methoxy-4-fluorobenzaldehyde, 1a) (440mg, 2.85mmol) In the dichloromethane solution, stirred at room temperature for 5 hours. The reaction solution was ice-cooled again, and cold water was slowly added to terminate the reaction, and then 5N hydrochloric acid solution was added dropwise until the pH was 1. The reaction solution was concentrated under reduced pressure, and the water and ethyl acetate were added to the residue, and the organic layer was separated. The organic layer was washed with saturated brine, dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. By silica gel column chromatography (elution solvent: The obtained residue was purified by n-hexane-ethyl acetate 4:1) to obtain 210 mg...
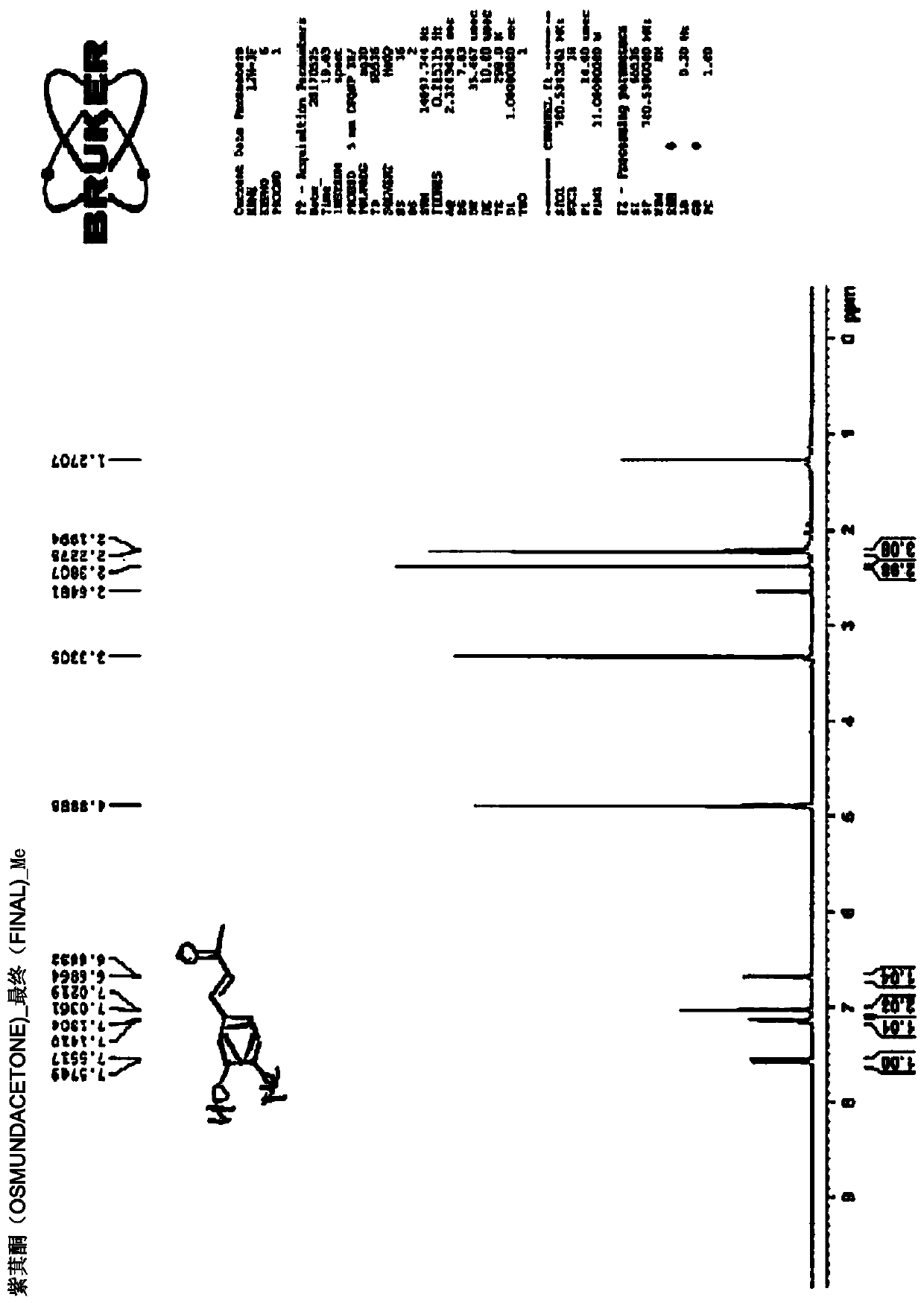
Embodiment 1-2
[0212] Synthesis of (E)-4-(3-hydroxy-4-methylphenyl)but-3-en-2-one (KP2)
[0213] 【Table 2】
[0214]
[0215] Under cooling, boron tribromide (1M dichloromethane solution, 5mL) was added dropwise to 3-methoxy-4-methylbenzaldehyde (3-methoxy-4-methylbenzaldehyde, 1b) (400mg, 2.66mmol) dichloromethane solution and stirred at room temperature for 5 hours.
[0216] The reaction solution was ice-cooled again, cold water was slowly added to terminate the reaction, and then 5N hydrochloric acid solution was added dropwise until the pH was 1. The reaction solution was concentrated under reduced pressure, water and ethyl acetate were added to the residue, and the organic layer was separated. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. Purify the obtained residue by silica gel column chromatography (elution solvent: n-hexane-ethyl acetate 4:1) to obtain 3-hydroxyl-4-methylben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com