Method for preparing linalool
A technology of linalool and dehydrolinalool, which is applied in hydrogenation preparation, chemical instruments and methods, preparation of hydroxyl compounds, etc., and can solve problems affecting the aroma of the final product linalool, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
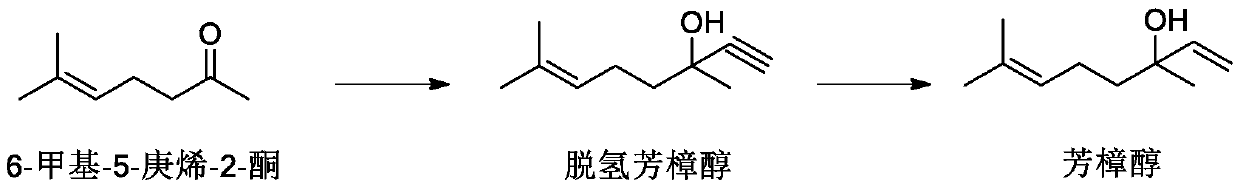
[0031] First, after replacing the ammonia gas in the autoclave, add liquid ammonia (481g, 28.3mol), start stirring at a set speed, feed acetylene (112L, 5.0mol) and 50wt% potassium hydroxide aqueous solution (substrate 3mol%), add 6 -Methyl-5-hepten-2-one (126.1g, 1.0mol), control the temperature at 30°C, and react for 2 hours after the feeding of 6-methyl-5-hepten-2-one is completed, then release the pressure Acetylene gas and ammonia gas. Add 60g of pure water to the reaction solution for extraction, keep the water phase, add 60g of pure water to the organic phase for extraction, and sample the organic phase.
[0032] GC detection reaction. Composition of organic phase reaction liquid: dehydrolinalool 98.43%, 6-methyl-5-hepten-2-one 1.36%, 6-methyl-5-hepten-2-one acetylenic diol 0.03%, others 1.31%. The conversion rate is 98.64%, and the selectivity is 98.79%.
[0033] The composition of the rectification raw material methyl heptenone ynylation reaction liquid: water con...
Embodiment 2
[0040]Firstly, after replacing the ammonia in the autoclave, add liquid ammonia (481g, 28.3mol), start stirring at a set speed, feed acetylene (67.2L, 3.0mol) and 50wt% potassium hydroxide aqueous solution (substrate 1mol%), add 6-Methyl-5-hepten-2-one (126.1g, 1.0mol), control the temperature at 50°C, after the 6-methyl-5-hepten-2-one feed is completed, react for 6h, and release the pressure Release acetylene gas and ammonia gas. Add 60g of pure water to the reaction solution for extraction, keep the water phase, add 60g of pure water to the organic phase for extraction, and sample the organic phase.
[0041] GC detection reaction. Composition of organic phase reaction liquid: dehydrolinalool 97.09%, 6-methyl-5-hepten-2-one 1.57%, 6-methyl-5-hepten-2-one acetylenic diol 0.01%, others 1.33%. The conversion rate is 98.43%, and the selectivity is 98.64%.
[0042] The composition of the rectification raw material methyl heptenone ynylation reaction liquid: water content 4.52%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com