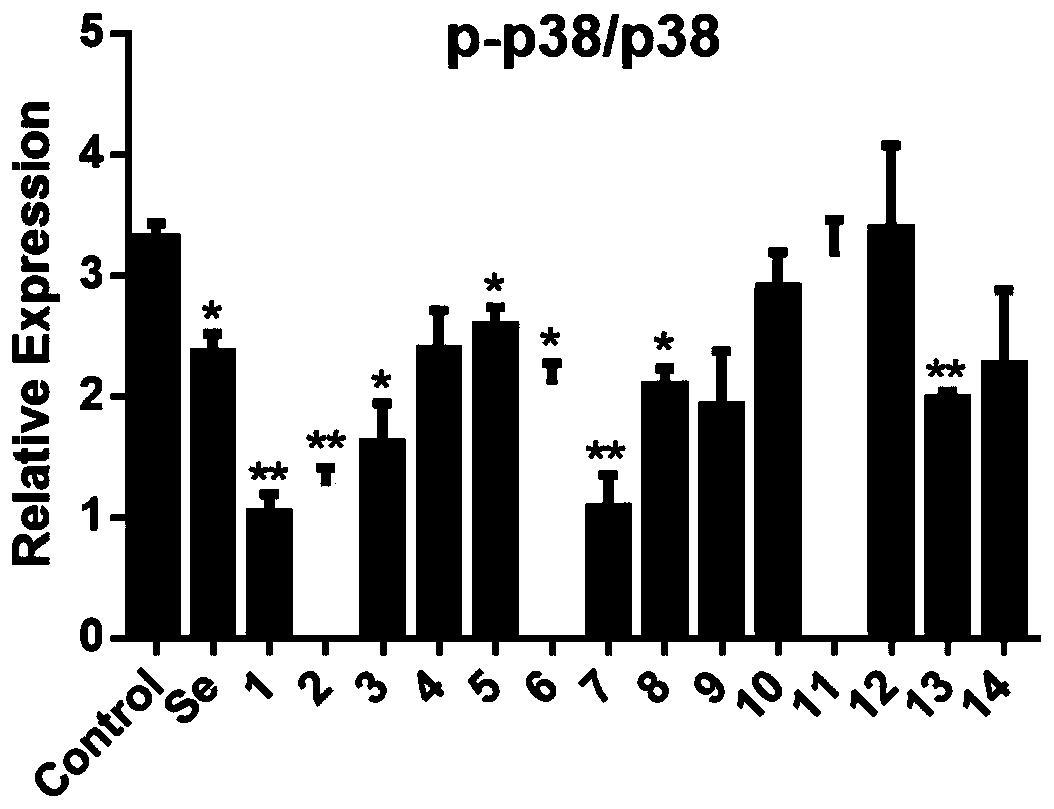
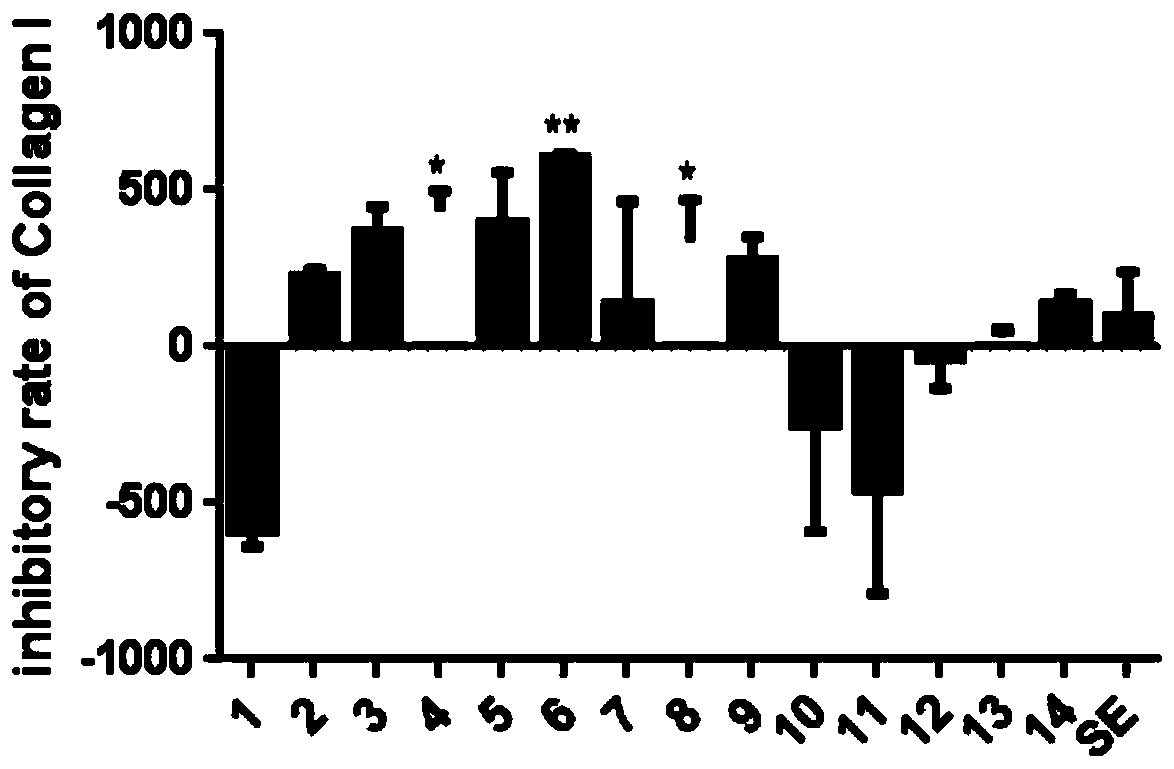
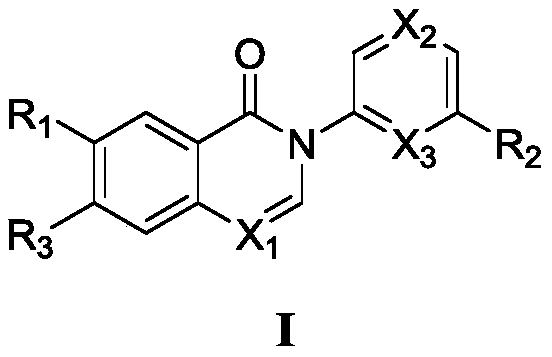
Apoptosis signal regulating kinase inhibitor and application thereof
A compound and selected technology can be used in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve problems such as fibrosis, extracellular matrix deposition, etc. Good inhibitory ability, low effect on normal cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of compound 1, the structural formula is as follows:
[0039]
[0040] Dissolve 6-bromopicolinic acid (50g, 0.249mol) in 300mL of methanol, slowly add 100mL of concentrated sulfuric acid dropwise to the reaction solution under stirring at 0°C, stir at 0°C for 30min after the dropwise addition, and then raise the temperature to 65°C, Reflux reaction for 3h. After the reaction was completed, it was cooled to room temperature, and then the reaction solution was poured into 3 L of ice water. The precipitated solid was filtered, washed with cold water, and dried to obtain 51 g of white crystalline solid, yield: 95%. Melting point: 67-78°C; 1H NMR (300MHz, DMSO) δ (ppm) 8.08 (dd, J = 1.51Hz, 1.39Hz, 1H), 7.94 (d, J = 1.51Hz, 1H), 7.92 (d, J =1.39Hz,1H),3.90(s,3H).MS(TOF)m / z:215.9[M+H]+.
Embodiment 2
[0042]The preparation of compound 2, structural formula is as follows:
[0043]
[0044] Dissolve 4-methyl-2-aminobenzonitrile (50g, 0.378mol) in 400mL dimethyl sulfoxide, add potassium carbonate (78.5g, 0.568mol) under stirring, and add to the reaction solution at 0°C Slowly add 200mL of 30% hydrogen peroxide solution dropwise into the solution, stir at 0°C for 1h after the dropwise addition, then move to room temperature for 4h. After the reaction was completed, 500ml of water was added to the reaction solution at 0°C, and then the precipitated solid was filtered, washed with cold water, and dried to obtain 52g of a light yellow solid, yield: 92%. Melting point: 87-93°C; 1H NMR (300MHz, CDCl 3 )δ(ppm)7.42(d,J=8.07Hz,1H),6.46(s,1H),6.29(d,J=8.07Hz,1H),2.15(s,3H).MS(TOF)m / z :151.1[M+H]+.
Embodiment 3
[0046] The preparation of compound 3, structural formula is as follows:
[0047]
[0048] Compound 2 (52g, 0.346mol) was dissolved in 300mL 1,2 dichloroethane, triethylamine (52.5g, 0.519mol) was added under stirring, and dicarbonate dicarbonate was slowly added to the reaction solution at 0°C Tert-butyl ester (151.0 g, 0.692 mol), after the dropwise addition, stirred at 0°C for 30 minutes, then raised the temperature to 60°C for 2 hours. After the reaction, the reaction solution was cooled to room temperature, concentrated to remove most of the solvent, and then the solid was dissolved in 100 ml of ethyl acetate, and 150 ml of petroleum ether was added to the solution at 0° C. for recrystallization. The precipitated solid was suction filtered, washed with petroleum ether, and the recrystallization operation was repeated twice. Dry to obtain 65g of yellow solid, yield: 75%. Melting point: 59-73°C; 1H NMR (300MHz, CDCl 3 )δ(ppm)8.26(s,1H),7.40(d,J=7.91Hz,1H),6.83(d,J=7.91...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com