Recombinant antibody of anti-human cardiac troponin I
A troponin and antibody technology, applied in the field of immunity, can solve the problems of poor affinity and low activity, and achieve the effect of high affinity and strong binding protein activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] This example provides an exemplary preparation method of a recombinant antibody against human cardiac troponin I.
[0121] S10, construction of expression plasmids:
[0122] In this embodiment, restriction endonuclease and Prime Star DNA polymerase were purchased from Takara Company;
[0123] MagExtractor-RNA extraction kit was purchased from TOYOBO;
[0124] BD SMART TM The RACE cDNA Amplification Kit was purchased from Takara;
[0125] The pMD-18T vector was purchased from Takara;
[0126] The plasmid extraction kit was purchased from Tiangen Company;
[0127] Primer synthesis and gene sequencing were completed by Invitrogen;
[0128] Secreting Anti-cTnI 12D2 monoclonal antibody is an existing hybridoma cell line, which is recovered for use.
[0129] S11, design and synthesis of primers:
[0130] 5' RACE upstream primers for amplification of heavy and light chains:
[0131] SMARTER II A Oligonucleotide:
[0132] 5'>AAGCAGTGGTATCAACGCAGAGGTACXXXXX<3';
[013...
Embodiment 2
[0152] Transient Transfection of Recombinant Antibody Expression Plasmids into CHO Cells and Identification of Antibody Activity in Expression Supernatant
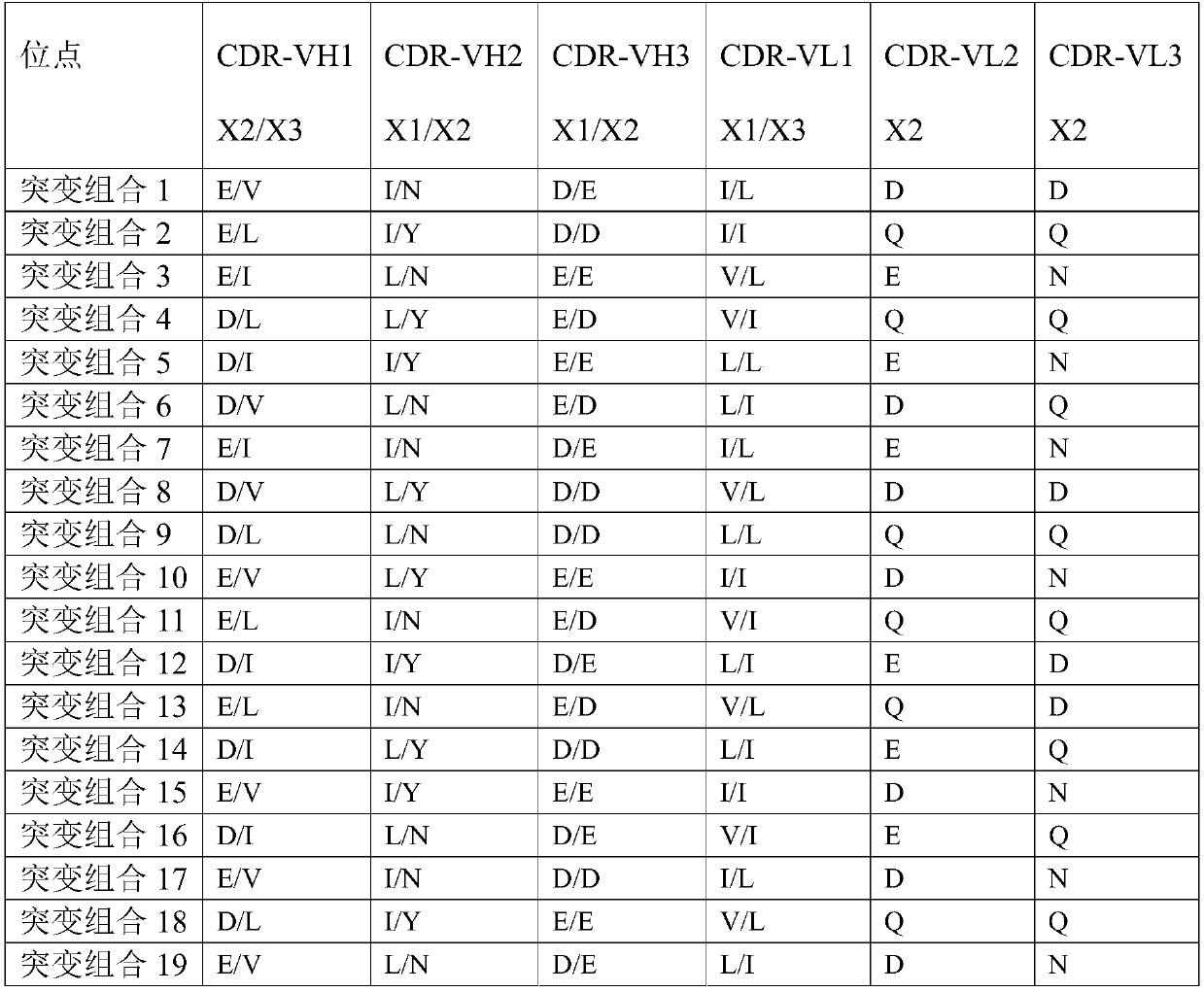
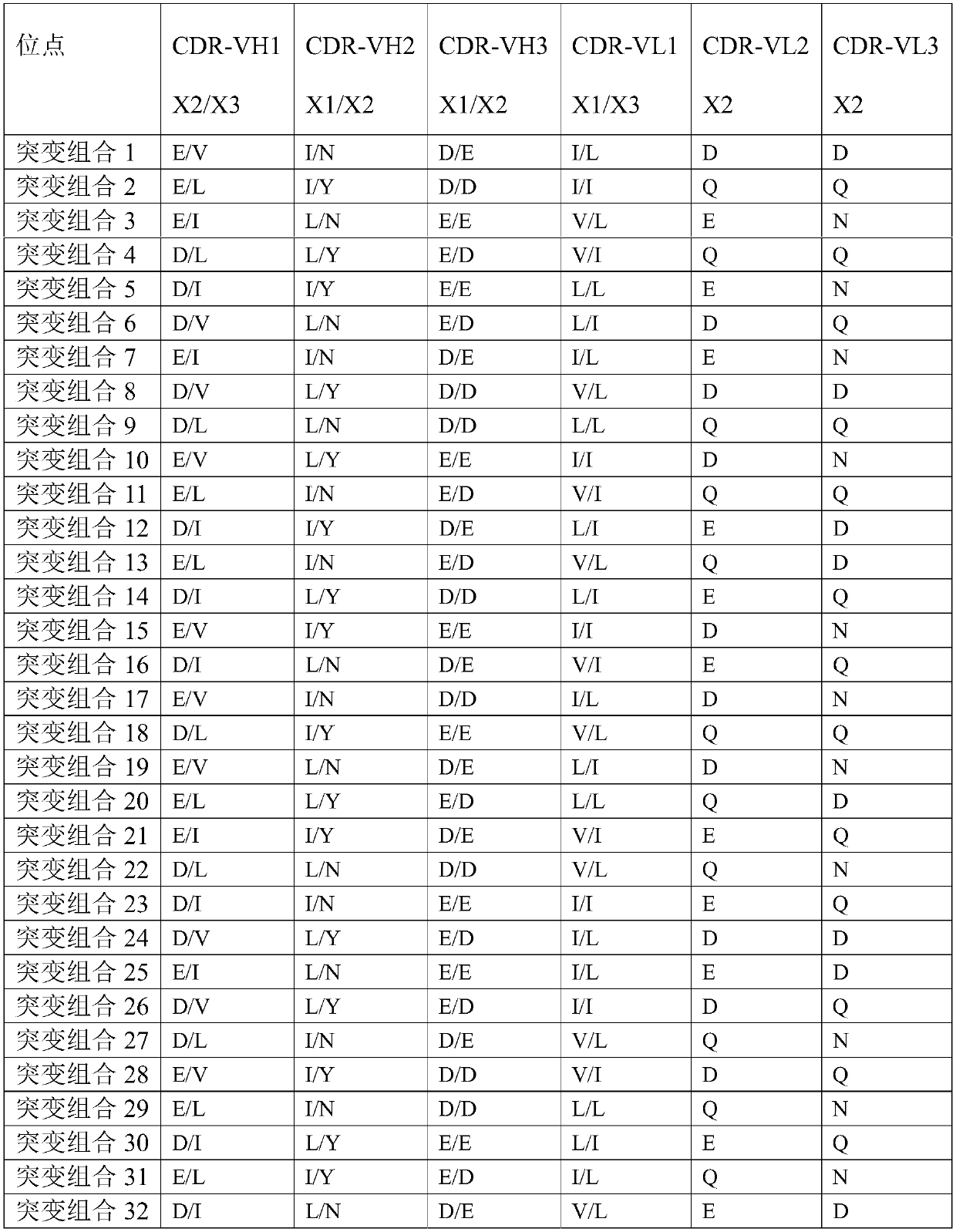
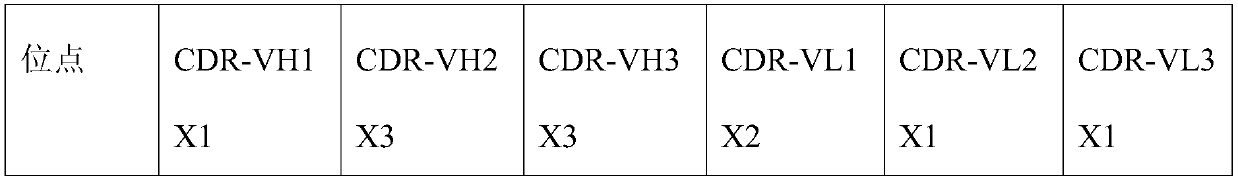
[0153] The plasmid was diluted to 400ng / ml with ultrapure water, and the CHO cells were adjusted to 1.43×10 7 cells / ml in a centrifuge tube, mix 100ul plasmid with 700ul cells, transfer to electroporation cup, electroporation, transfer to 10ml CD CHO AGT medium, culture in 37 degree shaker (8% CO 2 , amplitude 150); sampling every day to detect cell viability, when the cell viability is lower than 50%, centrifugal cell culture supernatant, the antibody obtained (has sequence such as light chain and heavy chain shown in SEQ ID NO:11 and 12 ). Take 4 μg of purified antibody for reducing SDS-PAGE.
[0154] After analysis, the complementarity determining region (WT) of the heavy chain:
[0155] CDR-VH1 is G-F(X1)-T-F-T-E(X2)-Y-N-V(X3)-H;
[0156] CDR-VH2 is Y-I(X1)-Y-P-N(X2)-N-G-I-S(X3)-G-Y-N-Q;
[0157] CDR-VH3 is R-D(X1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com