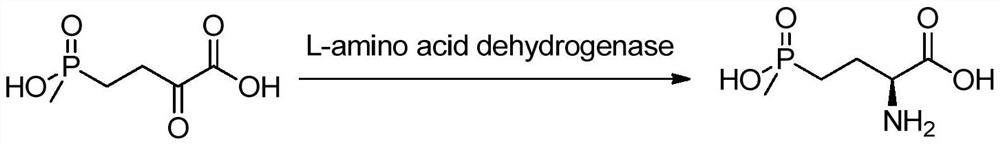
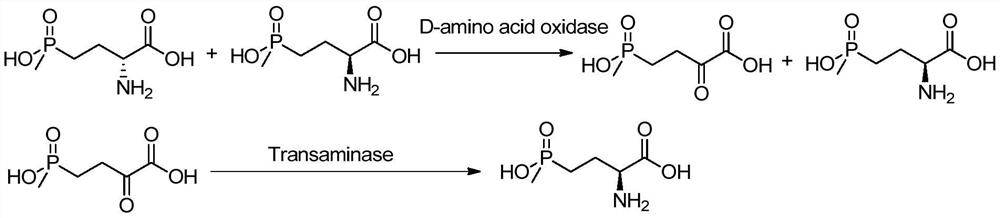
A d-amino acid oxidase mutant and its application
An amino acid and oxidase technology, applied in the direction of oxidoreductase, application, enzyme, etc., can solve the problems of low enzymatic activity, poor ammonium resistance, and poor enzyme activity stability of D-amino acid oxidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 wild-type D-amino acid oxidase (DAAO)
[0042] N from Rhodotorula sp.JG-1b retrieved from NCBI with GenBank accession number KWU45700 2 DAAO enzyme, fully synthetic wild-type (wt) N 2 DAAO enzyme gene, the gene synthesis company is Suzhou Jinweizhi Biotechnology Co., Ltd. (Floor C3, Bio-Nano Science and Technology Park, No. 218, Xinghu Street, Suzhou Industrial Park).
[0043] The synthesized wtN 2 DAAO enzyme gene connection plasmid pET28a (see J.Am.Chem.Soc., 2017, 139(32), 11241-11247 for specific methods), restriction sites NdeI and HindIII, transform the enzyme-linked vector into the host large intestine Bacillus BL21 competent cells; inoculate them into LB liquid culture based on 37°C, 200rpm shaker culture, until OD 600 When the temperature reaches about 0.8, take the bacterial solution and add sterile glycerol with a final concentration of 25%, and store it in a -80°C low-temperature refrigerator for later use.
[0044] The co...
Embodiment 2
[0047] Example 2 Construction of D-amino acid oxidase (DAAO) mutant library (position 211, position 213)
[0048] wtN as described in Example 1 2 On the basis of the DAAO sequence, the 54th and 58th positions (specifically N54V, F58Q) were mutated to obtain a mutated D-amino acid oxidase sequence, and the gene N was synthesized according to the sequence 2 DAAO (N54V, F58Q), the gene synthesis company is Suzhou Jinweizhi Biotechnology Co., Ltd. (Building C3, Bio-Nano Science and Technology Park, No. 218, Xinghu Street, Suzhou Industrial Park). Then introduce plasmid pET28a with NdeI and HindIII restriction sites to construct plasmid pET28a-N 2 DAAO (see J.Am.Chem.Soc., 2017, 139(32), 11241-11247 for plasmid construction methods). pET28a-N 2 DAAO was used as a template, and the target band was amplified by PCR.
[0049] Wherein, the primer sequence of designing PCR is constructed and designed for the mutant library that is mutated at the 211th and 213rd positions of the muta...
Embodiment 3
[0058] Example 3 High-throughput screening mutant library
[0059] Screen according to the following experimental steps:
[0060] The transformant obtained in Example 2 was inoculated into a 96-well plate for culture, and induced overnight at 30° C. with IPTG. Afterwards, the bacteria were harvested, cracked with bugbuster protein extraction reagent, and centrifuged to obtain the DAAO mutant enzyme solution.
[0061] Microplate reader detection method: take 100 μL of 100 mM substrate (racemic glufosinate-ammonium, purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.) with a pH of 8.0, and add 50 μL of chromogenic solution (containing 60 mg / mL of TBHBA (3 -hydroxy-2,4,6-tribromobenzoic acid) and 100 mg / mL of 4-AAP (4-aminoantipyrine)) and 25 μL of HRP (horseradish peroxidase, 0.1 mg / mL), and finally Add 25 μL of the above-mentioned DAAO mutant enzyme solution to obtain a 200 μL reaction system on a microtiter plate, and analyze it at 30° C. and pH 8.0. Record the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com