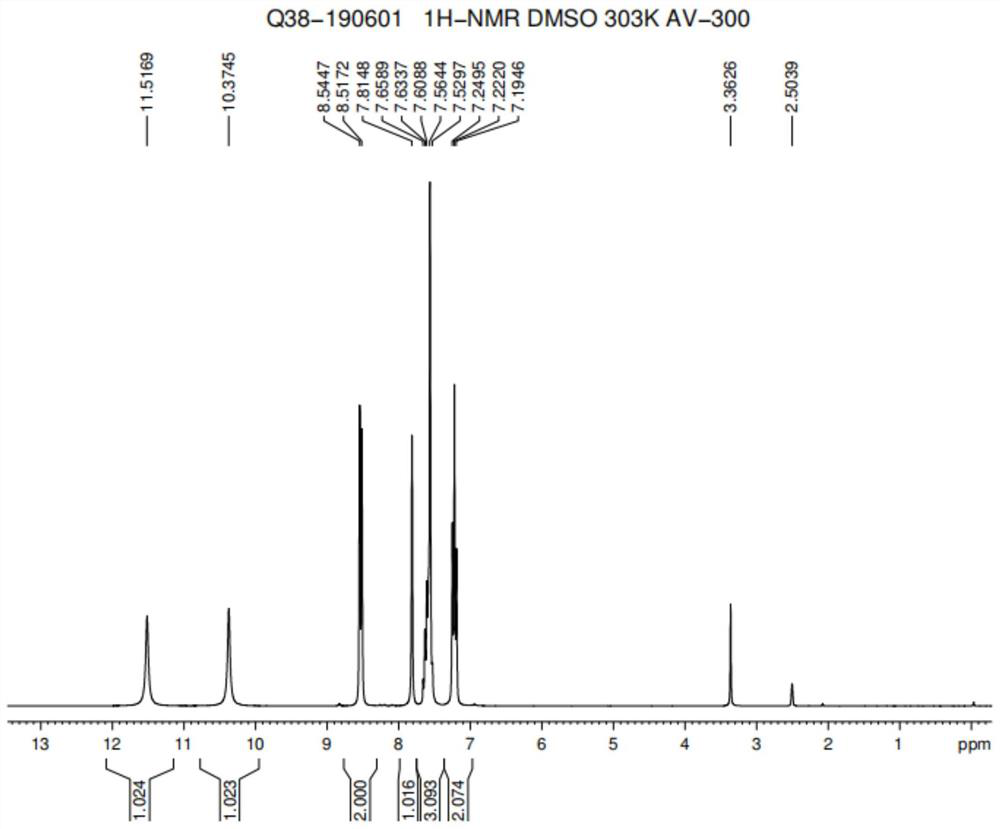
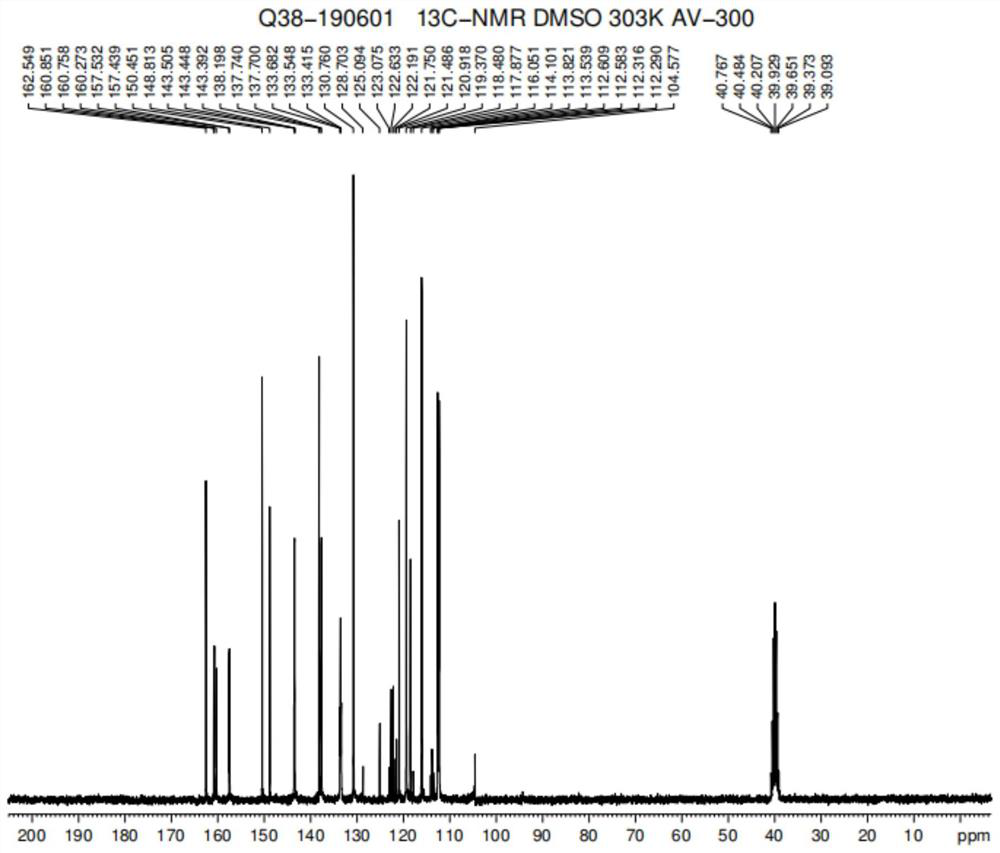
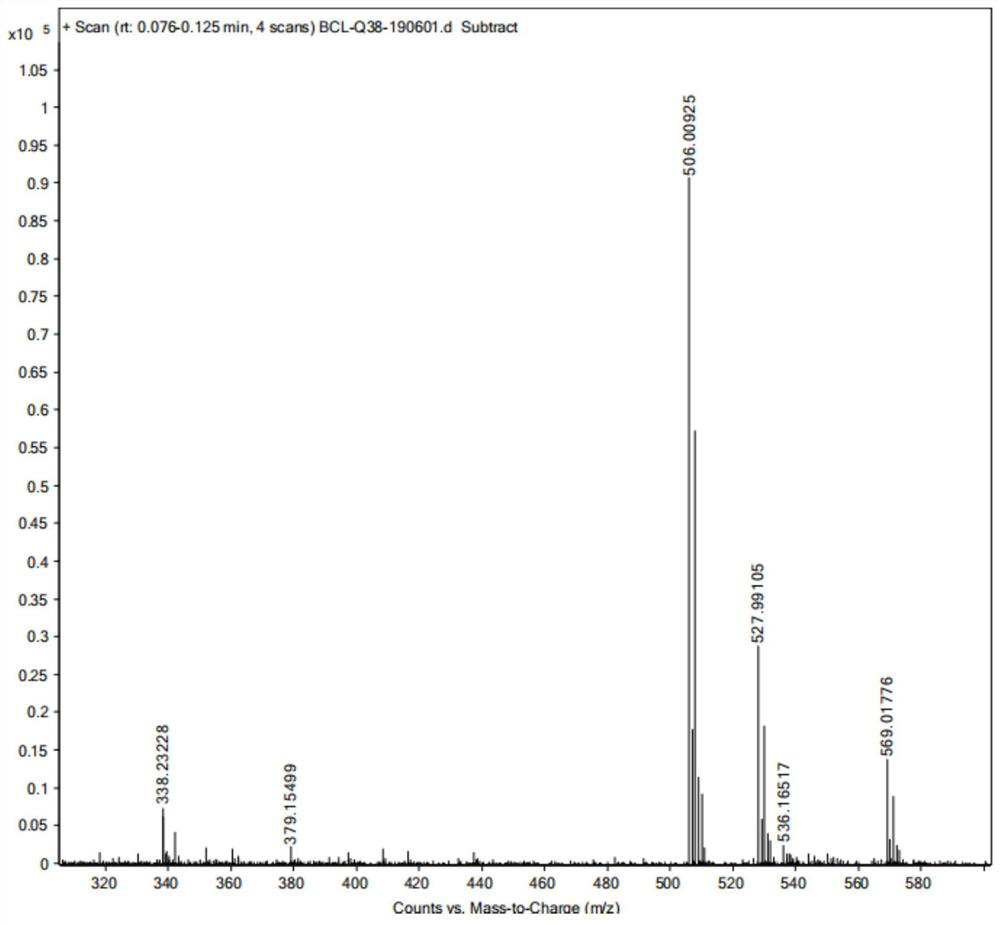
A kind of preparation method of imidazoram
A technology of imidacloprid and trifluoromethyl pyridine, applied in the field of preparation of imidacloprid, can solve the problems of expensive catalyst and complicated process steps, and achieve the effects of reducing energy consumption of products, simplifying processes, and reducing operation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Put 60ml of toluene and 15.7g of 0.1mol 2,6-difluorobenzamide into the reaction kettle, start to add 15.2g of 0.12mol oxalyl chloride dropwise at 25°C, after the dropwise addition, adjust the temperature to 40-50°C and keep it warm React for 1 hour, heat preservation is over, slowly raise the temperature to slight reflux, and heat preservation reaction at 100-115°C for 5-6 hours; cool down to 10-30°C to prepare the standby liquid formula Ⅰ;
[0030] (2) 13.7 g of 0.095 mol of 2-chloro-5-aminophenol, 10.6 g of potassium carbonate of 0.077 mol, and 60 ml of toluene were stirred at room temperature for 30 min in a dry and clean reactor to obtain formula II;
[0031] (3) Add the reserve liquid formula I dropwise to the formula II, control the temperature at 15-35°C, add 0.1g of tetrabutylammonium bromide and 1.5ml of DMF after the drop, and stir at the temperature of 15-35°C for 24 hours to obtain the formula III;
[0032] (4) Add 20.4 g of 0.095 mol of 2,3-dichloro-5-...
Embodiment 2
[0034] (1) Put 60ml of toluene and 15.7g of 0.1mol 2,6-difluorobenzamide into the reaction kettle, start to add 23.6g of 0.15mol oxalyl chloride dropwise at around 25°C, after the dropwise addition, adjust the temperature to 40-50°C Heat preservation reaction for 1 hour, heat preservation is over, slowly raise the temperature to slight reflux, and heat preservation reaction at 100-115°C for 5-6 hours; distill toluene and excess oxalyl chloride under reduced pressure, cool down to 10-30°C, and prepare the standby liquid formula I;
[0035] (2) 14.0 g of 0.097 mol of 2-chloro-5-aminophenol, 12.1 g of 0.088 mol of potassium carbonate, and 60 ml of toluene were dropped into a dry and clean reactor, and stirred at room temperature for 30 min to obtain formula II;
[0036] (3) Add the reserve liquid formula I dropwise to the formula II, and control the temperature at 15-35°C; add 0.1g of tetrabutylammonium bromide and 1.5ml of DMF after dropping, and stir at the temperature of 15-35°...
Embodiment 3
[0039] (1) Put 60ml of toluene and 15.7g of 0.1mol of 2,6-difluorobenzamide into the reaction kettle, start to drop 28.3g of 0.18mol of oxalyl chloride at around 25°C, after the dropwise addition, adjust the temperature to 40-50 ℃ heat preservation reaction for 1 hour, after the heat preservation is over, slowly raise the temperature to slight reflux, and heat preservation reaction at 100-115 ℃ for 5-6 hours; cool down to 10-30 ℃ to prepare the standby liquid formula Ⅰ;
[0040] (2) 14.3 g of 0.099 mol of 2-chloro-5-aminophenol, 13.8 g of 0.10 mol of potassium carbonate, and 60 ml of toluene were dropped into a dry and clean reactor, and stirred at room temperature for 30 min to obtain formula II;
[0041] (3) Add the reserve liquid formula I dropwise to the formula II, and control the temperature at 15-35°C; add 0.1g of tetrabutylammonium bromide and 1.5ml of DMF after dropping, and stir at the temperature of 15-35°C for 24 hours to obtain the formula III;
[0042] (4) Add 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com