Synthesis method of lifitegrast intermediate 5,7-dichloro-1,2,3,4-tetrahydroisoquinoline
A technology of tetrahydroisoquinoline and synthetic method, which is applied in the field of medicine and chemical industry, can solve the problems of unsuitability for industrial production, high Friedel-Crafts reaction temperature, and low reaction yield, and achieve mild conditions, readily available raw materials, and simple process route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]
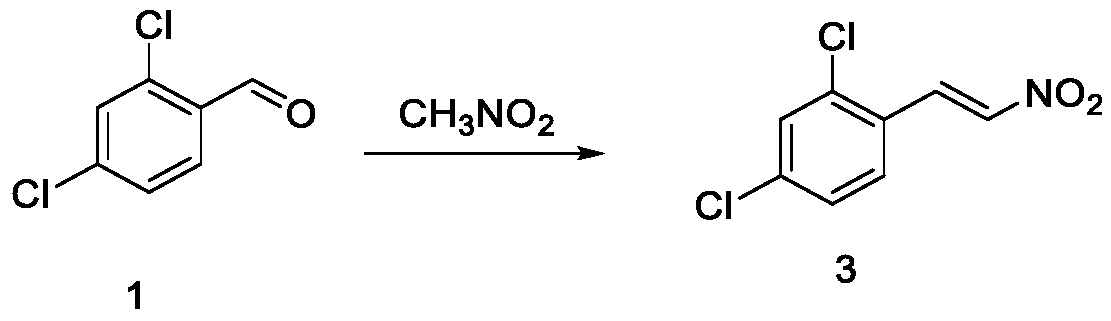
[0068] Add N,N-dimethylformamide (1093.8mL), potassium carbonate (51.8g, 0.375mol), 2,4-dichlorobenzaldehyde (43.8g, 0.25mol) into a four-necked flask, stir, and slowly drop Nitromethane (30.5 g, 0.5 mol) was added, and after the dropwise addition, the temperature was raised to 140° C., and the reaction was stirred for 5 h. After the completion of the reaction as monitored by TLC, the temperature of the reaction solution was slowly cooled to 10-20°C, a large amount of solids precipitated out, cold filtered, the filter cake was washed with water until the effluent was neutral, and a white solid was obtained, which was air-dried at 50-60°C to obtain compound 3 ( 43.8g, molar yield 80.4%, HPLC purity: 99.3%).
[0069] retention time (min) type Peak width (min) Peak area Peak height Peak area ratio% 2.753 BB 0.730 65.5202 9.0161 0.3058 4.162 BB 0.165 0.4704 0.1040 0.0022 5.508 BB 0.302 12.0522 2.6244 0.0562 6.703 B...
Embodiment 2
[0071]
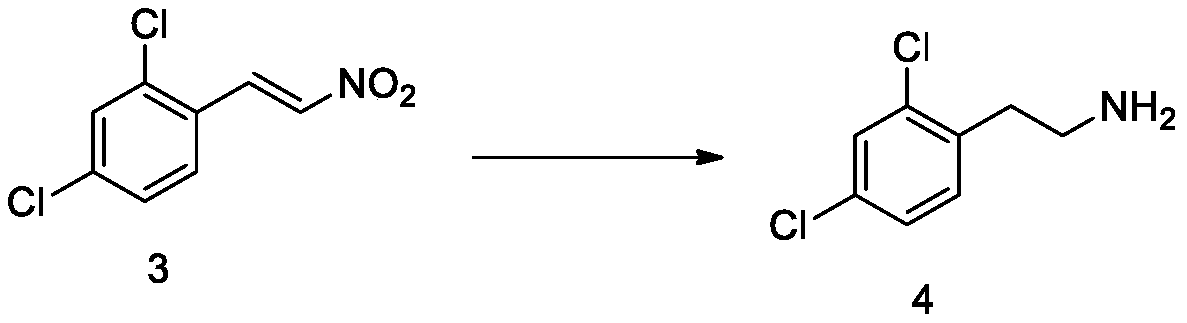
[0072] Add tetrahydrofuran (200mL) and lithium aluminum hydride (15.2g, 0.4mol) in sequence to the three-necked reaction flask, seal the reaction flask, replace with nitrogen three times, cool down to 0-5°C in an ice bath; The tetrahydrofuran solution of compound 3 obtained in Example 1 (compound 3, 43.8 g, 0.2 mol; tetrahydrofuran, 200 mL). After the dropwise addition, keep warm at 0-5°C for 12 hours. TLC monitors that the reaction is complete, add dropwise saturated sodium sulfate solution (100mL) to quench the reaction, filter, and concentrate the filtrate under reduced pressure to remove tetrahydrofuran, add ethyl acetate (200mL), water (100mL), separate layers, and the water layer TLC detects that there is no product, Discarded, the ethyl acetate layer was washed twice with saturated brine (100 mL), and the organic phase was concentrated to dryness under reduced pressure to obtain a brown oily compound 4 (34.7 g, molar yield: 90.9%, HPLC purity: 95.4%).
[00...
Embodiment 3
[0075]
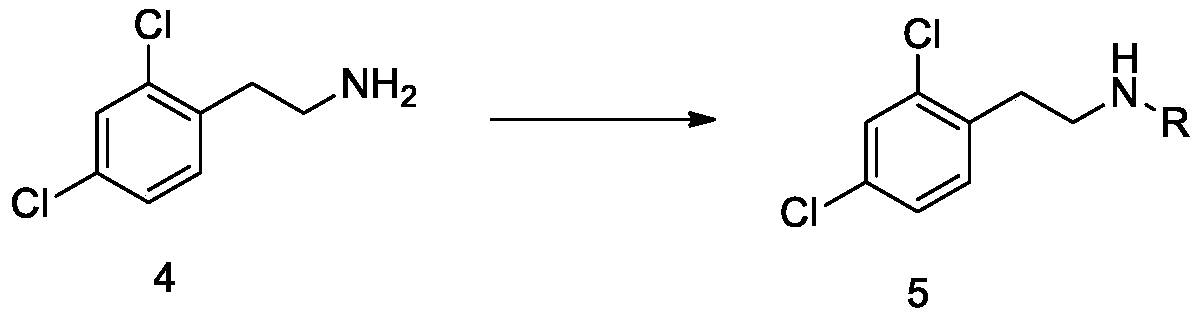
[0076] Add compound 4 (34.7g, 0.18mol) obtained in Example 2, dichloromethane (175mL), triethylamine (45.4g, 0.45mol) to the three-necked flask successively, replace with nitrogen three times, stir in an ice bath, and cool to 0 ~10°C; add acetic anhydride (22.4g, 0.22mol) dropwise, and control the internal temperature to 0~10°C. After the dropwise addition, keep the reaction for 3~5 hours. After TLC monitors the reaction is complete, add 100mL of water to quench, and separate the liquid. The aqueous phase was extracted once with 50 mL of dichloromethane, the organic phases were combined, washed twice with saturated brine (100 mL each time), the organic phase was collected, concentrated to dryness to obtain a white solid, added petroleum ether (60 mL), and beaten at room temperature for 1 h. After filtration, the filter cake was collected and dried under reduced pressure at 45-55°C to obtain a white solid, namely compound 5 (40.3 g, molar yield 95.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com