Device and method for removing hydrogen fluoride in fluorine gas
A technology of hydrogen fluoride and fluorine gas, which is applied in chemical instruments and methods, separation methods, gas treatment, etc., can solve the problems of fluorine gas liquefaction, etc., and achieve the effects of improving adsorption efficiency, reducing pulverization rate, and strengthening structural strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
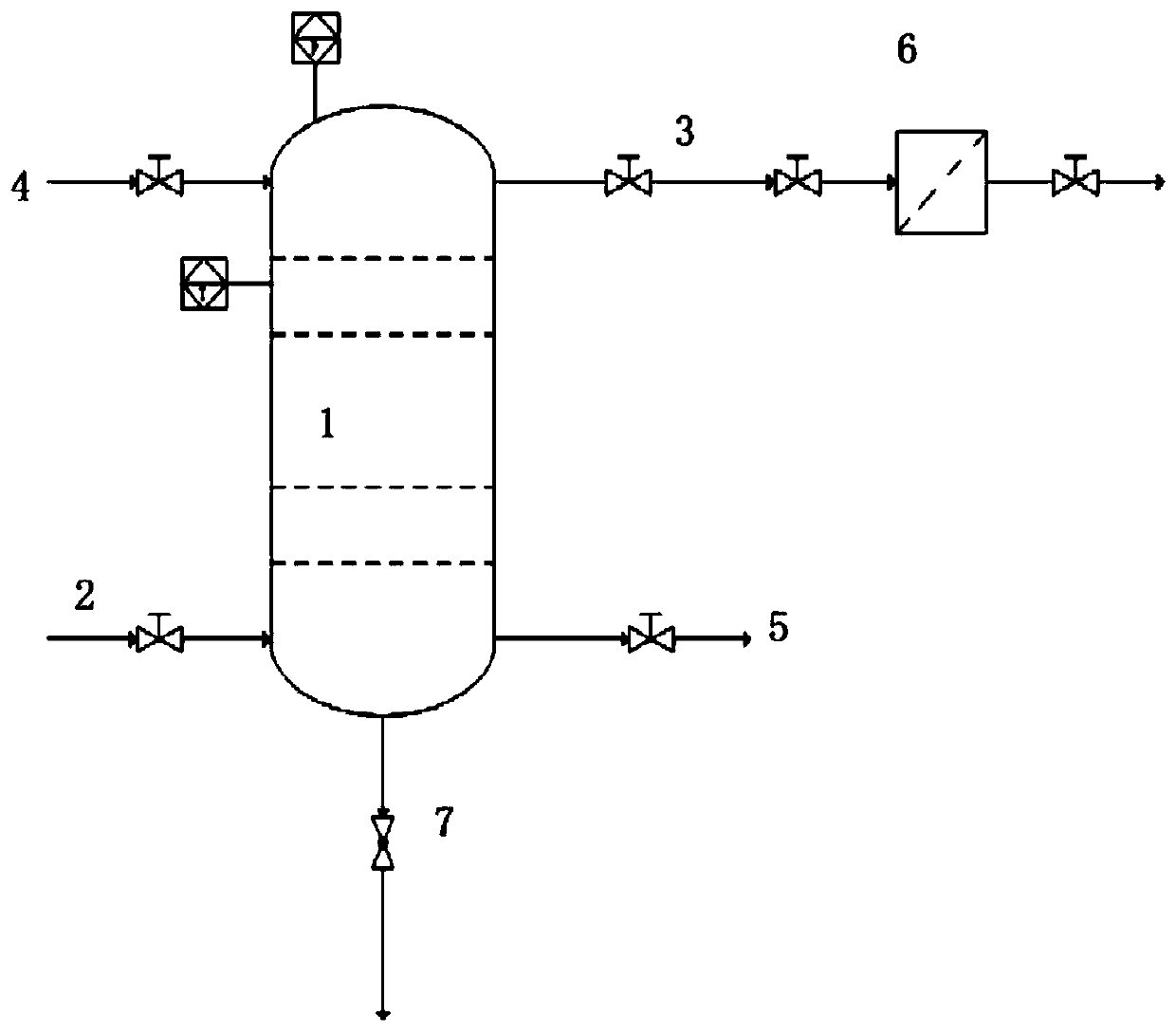
[0053] A method for removing hydrogen fluoride in fluorine gas, comprising the steps of:
[0054] Step 1, preparing NaF adsorption beads, the NaF adsorption beads use porous spheres as the skeleton, specifically including:
[0055] In step 1-1, NaF and HF are formed into a homogeneous molten mixture at 140° C. according to the molar ratio of 1:1.
[0056] In step 1-2, nickel is added to the mixture in step 1-1 to form porous pellets with a volume of about 0.2 cubic centimeters.
[0057] Step 1-3, lower the temperature to 60°C, wait until a semi-solidified state is formed, and take out the porous pellets with NaF attached.
[0058] Steps 1-4, drying the attached NaF porous beads at 100° C. to form sodium fluoride adsorption beads with a volume of about 0.5 cubic centimeters and porous beads as the skeleton.
[0059] Step 2, the fluorine gas with the HF content of 1200ppm produced by electrolysis is 1.5m 3 / h flow through F 2 The intake pipe 2 enters the adsorption tower equ...
Embodiment 2
[0062] A method for removing hydrogen fluoride in fluorine gas, comprising:
[0063] Step 1, prepare KF adsorption beads, NaF adsorption beads use a cylinder as the skeleton, specifically including:
[0064] Step 1-1, KF and HF are formed into a homogeneous mixture in a molten state at 60° C. according to a molar ratio of 1:6;
[0065] Step 1-2, adding Hastelloy alloy to the mixture in step 1-1 to make a cylinder with a volume of about 0.2 cubic centimeters;
[0066] Step 1-3, lower the temperature to 30°C, wait for a semi-solidified state, and take out the cylinder with KF attached;
[0067] In steps 1-4, the attached NaF cylinder is dried at 200° C. to form sodium fluoride adsorption beads with a volume of about 1.2 cubic centimeters.
[0068] Step 2, the fluorine gas with the HF content of 1400ppm produced by electrolysis in 4m 3 / h flow through F 2 The intake pipe 2 enters the adsorption tower equipped with KF adsorption beads from the lower part of the alkali metal ad...
Embodiment 3
[0071] A method for removing hydrogen fluoride in fluorine gas, comprising:
[0072] Step 1, preparing NaF adsorption beads, the NaF adsorption beads use porous beads as the skeleton, specifically including:
[0073] Step 1-1, forming a homogeneous mixture of NaF and HF in a molten state at 90° C. according to a molar ratio of 1:2.3;
[0074] Step 1-2, adding stainless steel to the mixture in step 1-1 to form porous pellets with a volume of about 1 cubic centimeter;
[0075] Step 1-3, lower the temperature to 50°C, wait until a semi-solidified state is formed, and take out the porous pellets with NaF attached;
[0076] In steps 1-4, the attached NaF porous balls are dried at 100° C. to form sodium fluoride adsorption balls with a volume of about 1.5 cubic centimeters and porous balls as the skeleton.
[0077] Step 2, the fluorine gas with the HF content of 1500ppm produced by electrolysis in 2.5m 3 / h flow through F 2 The intake pipe 2 enters the adsorption tower equipped ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com