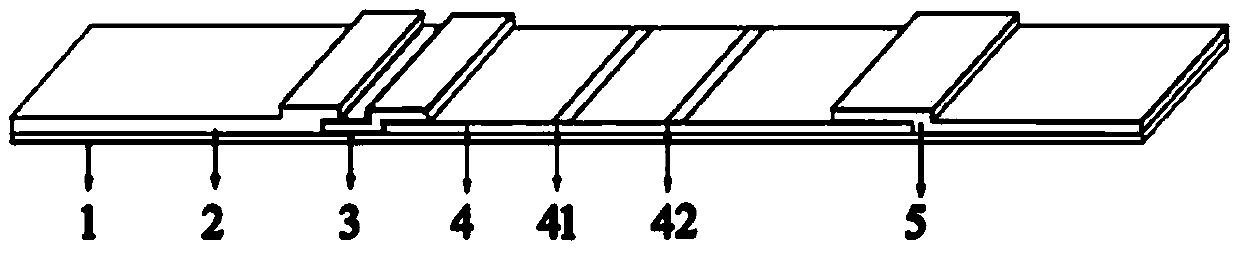
Colloidal gold immunochromatography kit for quickly detecting ovarian cancer tumor marker Legumain and preparation method of kit
A tumor marker, immunochromatography technology, applied in biological tests, measurement devices, analytical materials, etc., can solve problems such as obstacles to the popularization and promotion of early detection of ovarian cancer, high requirements for equipment and operators, and low diagnostic specificity of ovarian cancer. problems, to achieve the effect of small sample size, elimination of false positives, and mature preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0043] The present invention will be described below through specific embodiments, but the protection scope of the present invention is not limited to the following examples.
[0044] The Anti-Legumain-Ab1 and Anti-Legumain-Ab2 used in the kit of the present invention were purchased from Abcom Company, and the product numbers are ab183028 and ab232870 in sequence.
Embodiment 1
[0045] Example 1. Preparation of Colloidal Gold Kit for Rapid Detection of Legumain Anti-Legumain-Ab1 and Anti-Legumain-Ab2 are monoclonal antibodies against different epitopes of Legumain respectively.
[0046] (1) Preparation of colloidal gold
[0047] Take 80mL of ultrapure water in a clean Erlenmeyer flask and put it into a clean rotor, place it on a heatable magnetic stirrer and heat until bubbles come out evenly, then quickly add 1mL of 1% chloroauric acid solution, and heat to boiling Immediately add 1mL of 1.5% trisodium citrate, at this time the color of the solution in the Erlenmeyer flask changes from grayish black to wine red, continue heating for 7min, remove the Erlenmeyer flask, let it cool naturally at room temperature, and dilute to 100mL with a volumetric flask. Sealed for later use.
[0048] (2) Antibody labeling
[0049] Take 1 mL of the colloidal gold prepared in step (1), add potassium carbonate solution with a concentration of 0.1 mol / L, and adjust the...
Embodiment 2
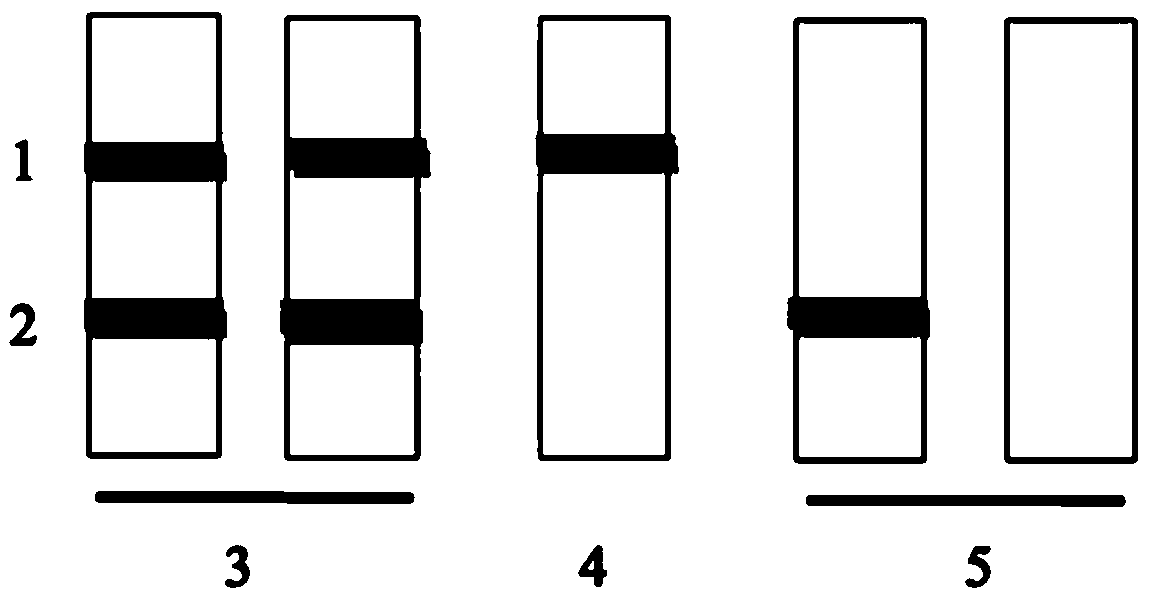
[0064] Embodiment 2, the method for judging the result of the colloidal gold-labeled anti-Legumain antibody kit
[0065] The result evaluation method of Legumain rapid detection kit is as follows: figure 2 As shown, when both the C quality control band 1 and the T detection band 2 are colored, the detected sample contains Legumain above the sensitivity (corresponding to figure 2 Middle 3); when the C quality control band 1 develops color, but the T detection band 2 does not develop color, it means that the tested sample does not contain or contain Legumain below the sensitivity (corresponding to figure 2 Middle 4); if the C quality control band 1 does not develop color, no matter whether the T detection band 2 develops color or not, the detection effect is invalid (corresponding to figure 2 Middle 5).
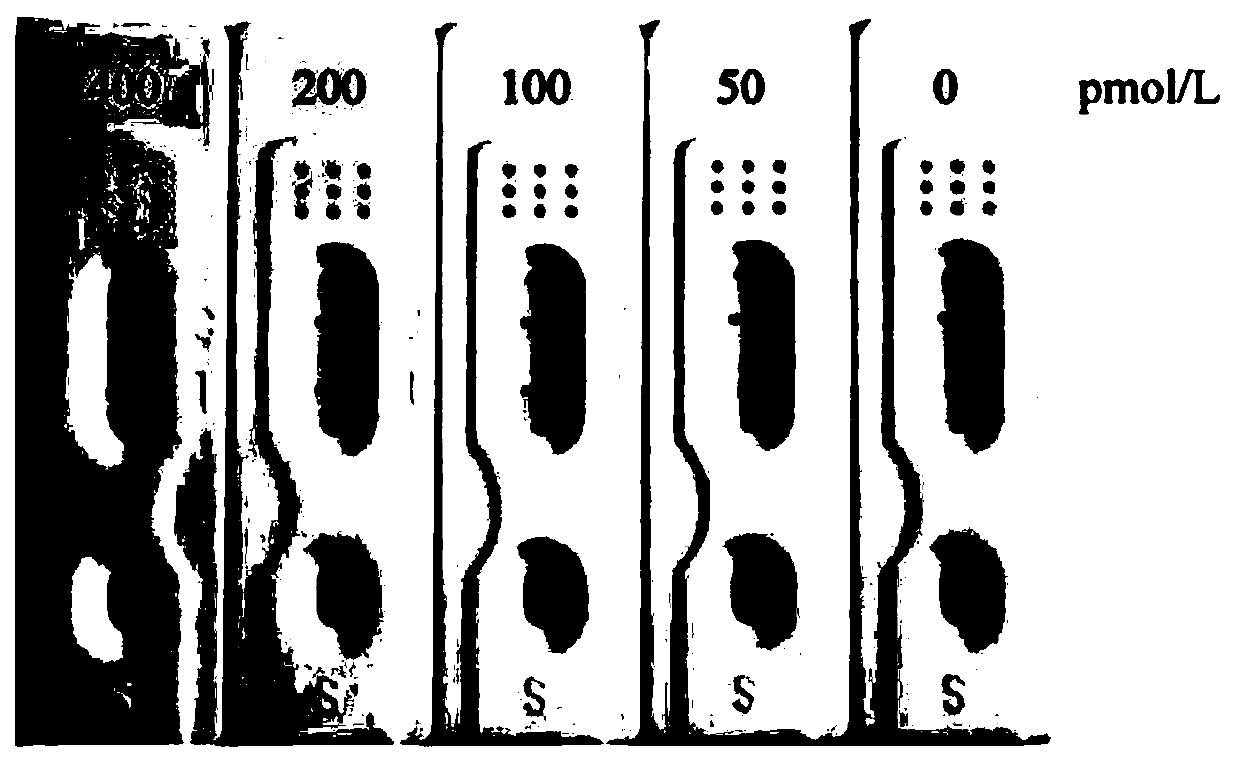
[0066] Performance testing experiment of Legumain colloidal gold rapid detection kit:
[0067] 1. Sensitivity test Dilute the Legumain standard with negative human serum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com