Method for synthesizing pentaerythritol tetraglycidyl ether
A technology of glycidyl ether and pentaerythritol tetraglycidyl ether, which is applied in the field of synthesis of pentaerythritol tetraglycidyl ether, can solve the problems of incomplete reaction, uncontrollable reaction, low yield, etc., to increase yield, reduce product color and hydrolyze chlorine, The effect of promoting a complete response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
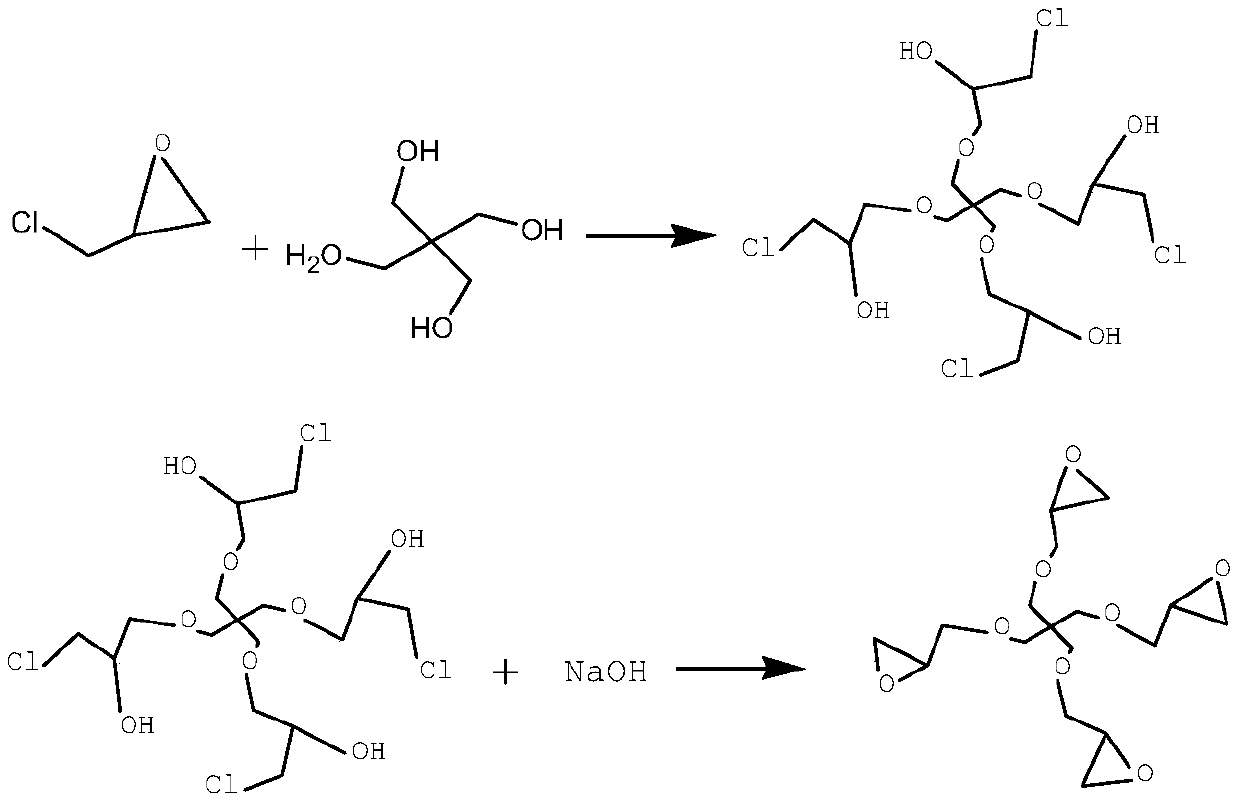
[0020] Weigh 34g (0.25mol) of pentaerythritol and put it into a four-neck flask with stirring, add 102g of dioxane, heat up while stirring, and add a composite catalyst (boron trifluoride etherate complex and tetrachloromethane) to 90-95°C Tin) were 0.60g and 1.50g respectively, and 115.6g (1.25mol) of epichlorohydrin was added dropwise, and kept for 2h after dripping for 4h. The above solution was put into a distillation flask to remove the solvent, the temperature was controlled below 110°C, the holding time was 30min, and the vacuum degree was kept above 0.095MPa. Add 252 g of toluene to the intermediate after solvent removal, keep stirring, add 0.60 g of benzyltriethylammonium bromide to it after raising the temperature to 40-45 ° C, and add 22.5 g of solid alkali sodium hydroxide (0.563 mol) within 2 hours , added in four times with an interval of 30 minutes; then the temperature was raised to 60-65° C., 70.3 g (0.563 mol) of 32% liquid caustic soda was dripped within 1 h...
Embodiment 2
[0022] Weigh 34g (0.25mol) of pentaerythritol and put it into a four-neck flask with stirring, add 102g of dioxane, heat up while stirring, and add a composite catalyst (boron trifluoride etherate complex and tetrachloromethane) to 90-95°C Tin) were 0.41g and 1.03g, respectively, 69.4g (0.75mol) of epichlorohydrin was added dropwise, and the temperature was kept for 2h after 4h. The above solution was put into a distillation flask to remove the solvent, the temperature was controlled below 110°C, the holding time was 30min, and the vacuum degree was kept above 0.095MPa. Add 252g of chloroform to the intermediate after solvent removal, stir continuously, add 0.41g of benzyltriethylammonium bromide to it after heating up to 40-45°C, and add 12.5g (0.31mol) of solid alkali sodium hydroxide within 2h , added in four times with an interval of 30 minutes; then the temperature was raised to 60-65°C, and 39.1 g (0.31 mol) of liquid caustic soda was dripped within 1 hour and kept for 2...
Embodiment 3
[0024] Weigh 34g (0.25mol) of pentaerythritol and put it into a four-necked flask with stirring, add 102g of chloroform, raise the temperature while stirring, and raise the temperature to 90-95°C, add 1.31g of catalyst boron trifluoride, and dropwise add 185.0g of epichlorohydrin (2.0mol), after 4 hours of dripping, keep warm for 2 hours. The above solution was put into a distillation flask to remove the solvent, the temperature was controlled below 100°C, the holding time was 30min, and the vacuum degree was kept above 0.095MPa. Add 252 g of ethylene dichloride to the intermediate after solvent removal, stir continuously, add 0.88 g of benzyltriethylammonium bromide to it after raising the temperature to 40-45 ° C, and add 37.5 g of solid alkali sodium hydroxide within 2 hours ( 0.94mol), added in four times with an interval of 30min; then the temperature was raised to 60-65°C, 117.2g (0.94mol) of liquid caustic soda was dripped within 1h and kept for 2h. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com