Bifidobacterium animalis and application of compound bacterium preparation prepared from bifidobacterium animalis in preparation of medicine for treating or preventing avian influenza virus infection
A technology of animal bifidobacteria and avian influenza virus, which is applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc. Lost, improve survival rate, increase the effect of clearing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of the probiotic preparation of anti-H7N9 avian influenza virus infection:
[0040] 1. Preparation of single bacterial preparations of Bifidobacterium animalis and Bifidobacterium pseudolongum
[0041] Bifidobacterium animalis ATCC 25527 and Bifidobacterium pseudolongum ATCC 25526. Resuspend the lyophilized powder of the strain with sterile PBS, pick an appropriate amount of bacterial liquid with an inoculation loop, streak and rejuvenate it on the MRS solid plate medium, and culture it under anaerobic conditions at 37°C for 24-48 hours. Streak culture the rejuvenated Bifidobacterium animalis and Bifidobacterium pseudolongum in MRS respectively, and culture them under anaerobic conditions at 37°C for 24 hours, wash the bacterial lawn on the MRS medium plate with PBS, and collect the bacterial suspension , centrifuge at 4°C and 5000rpm for 10 minutes, discard the supernatant, resuspend the collected Bifidobacterium animalis and Bifidobacterium pse...
Embodiment 2
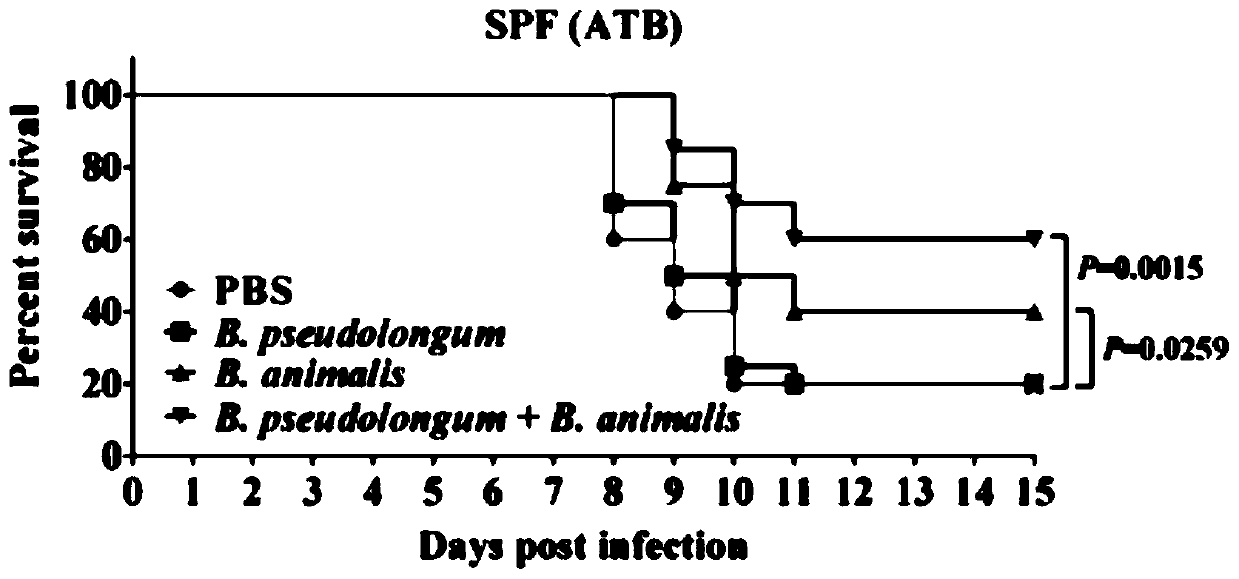
[0046] Effect of the probiotic preparation prepared in Example 1 on mouse mortality and body weight after H7N9 avian influenza virus infection
[0047] 1. Effects on Mortality and Body Weight of SPF Mice
[0048] The experimental animals were 8-week-old female C57BL / 6 specific pathogen-free (SPF) mice, a total of 80 were randomly divided into 4 groups, 20 in each group, and all animals were raised in a biosafety level 3 laboratory (ABSL3) . The first group is the control group fed with sterile PBS, 100ul per mouse; the second group is fed with Bifidobacterium pseudolongum (Bifidobacterium pseudolongum) ATCC 25526 single bacteria preparation group, 100ul per mouse; the third group is The single-bacteria preparation group of Bifidobacterium animalis ATCC 25527 was fed with 100ul per mouse; the fourth group was fed with the compound preparation of Bifidobacterium animalis ATCC 25527 and Bifidobacterium pseudolongum ATCC 25526 Group, 100ul per mouse.
[0049] Antibiotic treatme...
Embodiment 3
[0064] The effect of the probiotic preparation prepared in Example 1 on the influenza virus content in the lungs of mice after H7N9 avian influenza virus infection, the impact on the lung tissue structure of mice, and the impact on the cytokines in the lungs and the cytokines in the blood
[0065] 96 SPF mice were randomly divided into 4 groups, 24 in each group. The first group was fed with sterile PBS control group, 100ul per mouse; the second group was fed with Bifidobacterium pseudolongum (Bifidobacterium pseudolongum) ATCC 25526 single-bacteria preparation group, 100ul per mouse; the third group was fed with Bifidobacterium animalis (Bifidobacterium animalis) ATCC25527 single-bacteria preparation group, 100ul per mouse; the fourth group was fed with Bifidobacterium animalis ) ATCC 25527 and Bifidobacterium pseudolongum (Bifidobacterium pseudolongum) ATCC 25526 compound preparation group, 100ul per mouse.
[0066] As in Example 2, all SPF mice were raised in a biosafety le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com