Preparation method of porous NH2-UiO-66-d/indium zinc sulfide composite visible-light-induced photocatalyst
A technology of nh2-uio-66-d and nh2-uio-66, which is applied in the direction of organic compound/hydride/coordination complex catalyst, catalyst activation/preparation, physical/chemical process catalyst, etc., can solve hydrothermal stability In order to achieve the effect of improving photocatalytic hydrogen production activity, high repeatability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
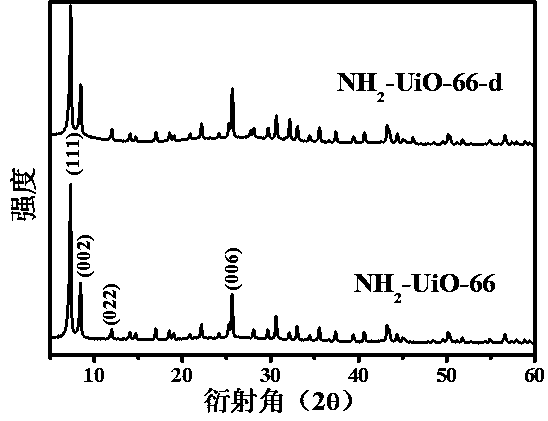
[0022] (1) NH 2 -Preparation of UiO-66: Dissolve 0.2332 g of zirconium chloride and 0.1812 g of 2-aminoterephthalic acid in 50 mL of N,N-dimethylformamide, stir and dissolve the solution, and transfer the solution to polytetrafluoroethylene In vinyl lining, solvothermal reaction at 90°C for 24 h, after the hydrothermal kettle was cooled to room temperature, the product was centrifuged and washed with N,N-dimethylformamide and anhydrous methanol to obtain NH 2 -UiO-66.
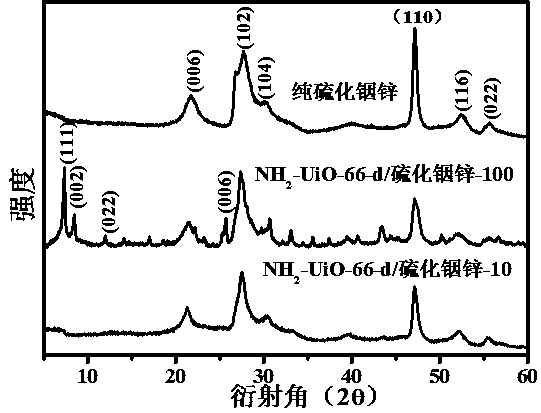
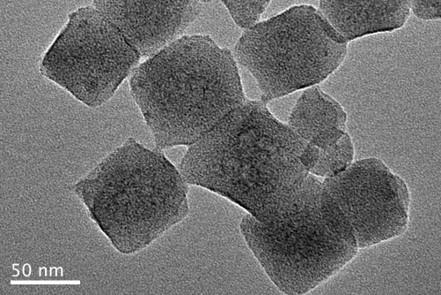
[0023] (2) NH 2 - Preparation of UiO-66-d: Dissolve 50 mg of silver nitrate and 50 mg of potassium persulfate in 20 mL of acetonitrile. After ultrasonic dissolution, the prepared 150 mg of NH 2 - Add UiO-66 to the above solution, put it in an oil bath at 150°C for 40 minutes, then quickly put the reactor into the ice-water mixture, after a period of time, use deionized water and ethanol as detergents to wash the product centrifugally, get porous NH 2 -UiO-66, which is NH 2 -UiO-66-d.
[0024] (3) NH 2 - ...
Embodiment 2
[0026] (1) NH 2 -Preparation of UiO-66: Dissolve 0.2332 g of zirconium chloride and 0.1812 g of 2-aminoterephthalic acid in 50 mL of N,N-dimethylformamide, stir and dissolve the solution, and transfer the solution to polytetrafluoroethylene In vinyl lining, solvothermal reaction at 90°C for 24 h, after the hydrothermal kettle was cooled to room temperature, the product was centrifuged and washed with N,N-dimethylformamide and anhydrous methanol to obtain NH 2 -UiO-66.
[0027] (2) NH 2 - Preparation of UiO-66-d: Dissolve 50 mg of silver nitrate and 50 mg of potassium persulfate in 20 mL of acetonitrile. After ultrasonic dissolution, the prepared 150 mg of NH 2 - Add UiO-66 to the above solution, put it in an oil bath at 150°C for 40 minutes, then quickly put the reactor into the ice-water mixture, after a period of time, use deionized water and ethanol as detergents to wash the product centrifugally, get porous NH 2 -UiO-66, which is NH 2 -UiO-66-d.
[0028] (3) NH 2 - ...
Embodiment 3
[0030] (1) NH 2 -Preparation of UiO-66: Dissolve 0.2332 g of zirconium chloride and 0.1812 g of 2-aminoterephthalic acid in 50 mL of N,N-dimethylformamide, stir and dissolve the solution, and transfer the solution to polytetrafluoroethylene In vinyl lining, solvothermal reaction at 90°C for 24 h, after the hydrothermal kettle was cooled to room temperature, the product was centrifuged and washed with N,N-dimethylformamide and anhydrous methanol to obtain NH 2 -UiO-66.
[0031] (2) NH 2 - Preparation of UiO-66-d: Dissolve 50 mg of silver nitrate and 50 mg of potassium persulfate in 20 mL of acetonitrile. After ultrasonic dissolution, the prepared 150 mg of NH 2 - Add UiO-66 to the above solution, put it in an oil bath at 150°C for 40 minutes, then quickly put the reactor into the ice-water mixture, after a period of time, use deionized water and ethanol as detergents to wash the product centrifugally, get porous NH 2 -UiO-66, which is NH 2 -UiO-66-d.
[0032] (3) NH 2 - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com