Indole compound and application and preparation method thereof
A compound, indole technology, applied in the field of medicine, can solve the problems of lack of effective tumor treatment, anti-tumor drugs can not meet the treatment and other problems, and achieve significant effects, anti-cancer effects, and good inhibitory effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
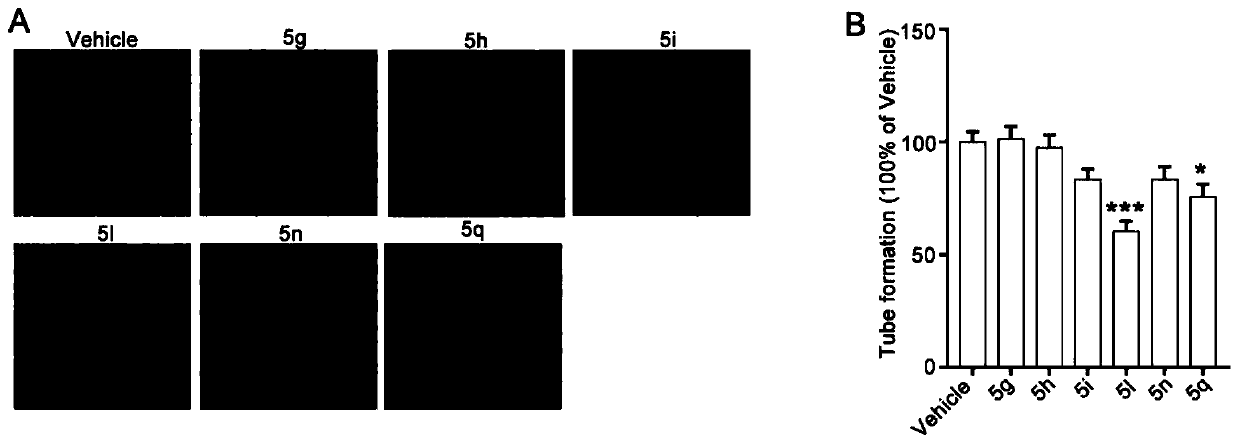
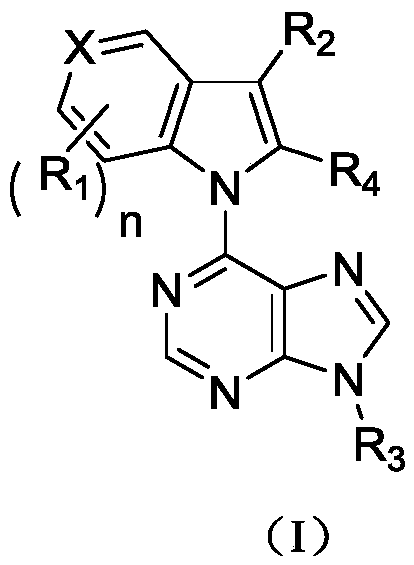
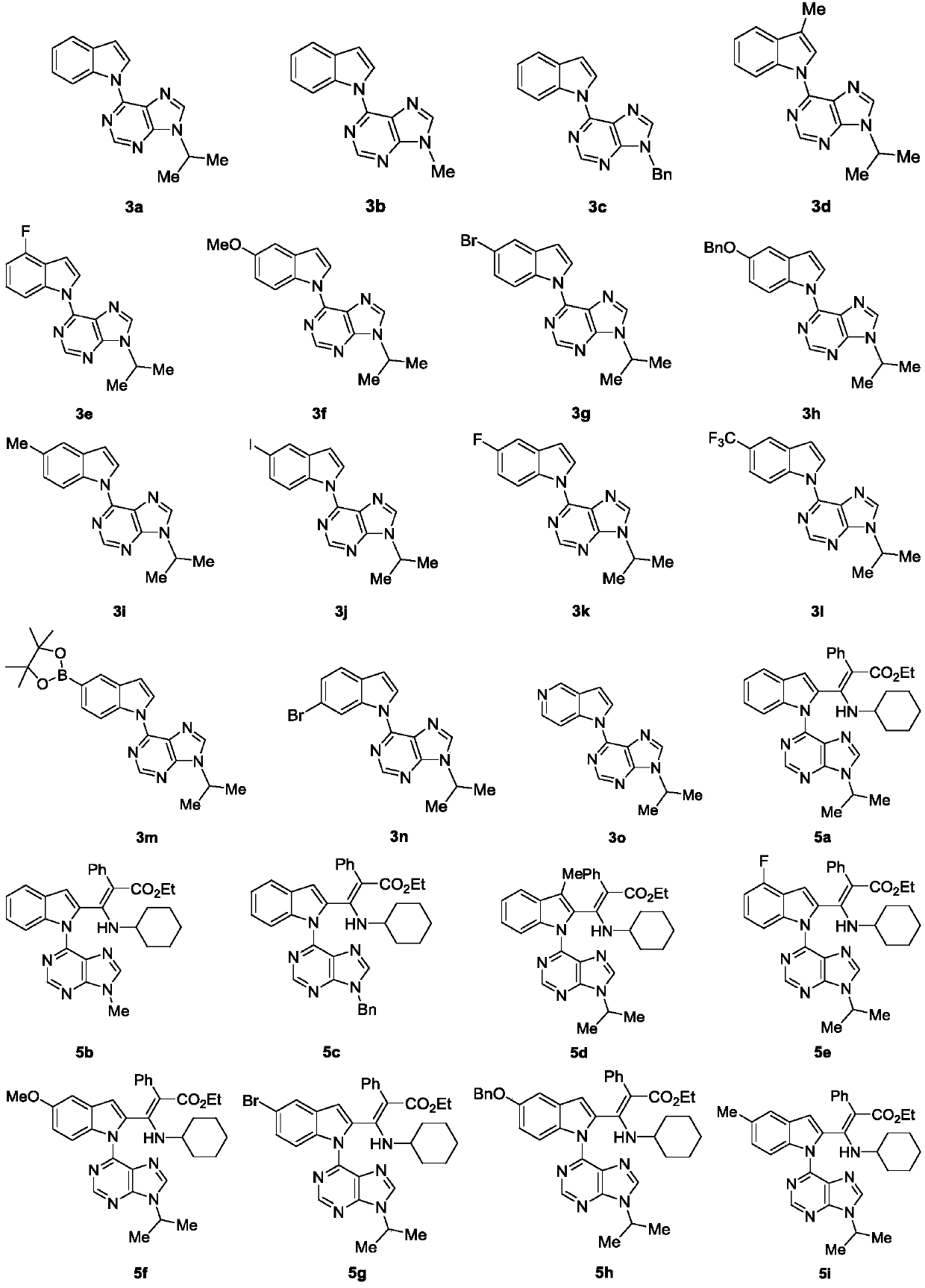
Image
Examples
Embodiment 1
[0096] The synthesis of embodiment 1 compound 3a
[0097]
[0098]Take a 100mL reaction bottle, evacuate it, fill it with nitrogen, and repeat the operation three times, then add 2a (421mg, 3.6mmol) to the bottle, then add DMF (30mL), stir at 0°C, and slowly add NaH in batches (60% distribution in mineral oil, 180 mg, 4.5 mmol) into the stirred solution. After stirring at 0°C for 30 minutes, 1a (590mg, 3.0mmol) was added, and the reaction was stirred at room temperature for 20 hours, then 100mL of water was added to quench the reaction, extracted with ethyl acetate (50mL×3), and the organic phase was washed with Dry over anhydrous sodium sulfate. After filtration, the filtrate was spin-dried (the solvent was evaporated under reduced pressure), and separated by silica gel column chromatography (petroleum ether / ethyl acetate=5 / 1) to obtain the yellow solid product 3a (749 mg, 90%). The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ=9.18(...
Embodiment 2
[0099] The synthesis of embodiment 2 compound 3b
[0100]
[0101] According to the method described in Example 1, 2a (421mg, 3.6mmol) was added to a 100mL reaction flask under nitrogen atmosphere, then DMF (30mL) was added, stirred at 0°C, and NaH was slowly added in batches (in Distribute 60% in mineral oil, 180 mg, 4.5 mmol) into the stirred solution. After stirring at 0°C for 30 minutes, 1b (497mg, 3.0mmol) was added, and the reaction was stirred at room temperature for 20 hours, then 100mL of water was added to quench the reaction, extracted with ethyl acetate (50mL×4), and the organic phase was washed with Dry over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column chromatography to obtain product 3b (523 mg, 70%) as a yellow solid. The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ=9.11(d, J=2.9Hz, 1H), 9.03(d, J=8.3Hz, 1H), 8.79(s, 1H), 7.86(s, 1H), 7.62(d, J=7.6Hz, 1H), 7.36 (t, J = 7.7H...
Embodiment 3
[0102] The synthesis of embodiment 3 compound 3c
[0103]
[0104] According to the method described in Example 1, 2a (421mg, 3.6mmol) was added to a 100mL reaction flask under nitrogen atmosphere, then DMF (30mL) was added, stirred at 0°C, and NaH was slowly added in batches (in Distribute 60% in mineral oil, 180 mg, 4.5 mmol) into the stirred solution. After stirring at 0°C for 30 minutes, 1c (732 mg, 3 mmol) was added, and the reaction was stirred at room temperature for 20 hours to obtain the product 3c (580 mg, 60%) as a white solid. The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ=9.17(d, J=3.7Hz, 1H), 9.09(d, J=8.5Hz, 1H), 8.86(s, 1H), 7.94(s, 1H), 7.65(d, J=7.7Hz, 1H), 7.40–7.29 (m, 7H), 6.80 (d, J=3.6Hz, 1H). 13 CNMR (100MHz, CDCl 3 ) δ = 152.8, 152.3, 150.0, 142.3, 136.2, 135.3, 130.8, 129.2, 128.7, 128.6, 127.9, 124.0, 123.0, 122.3, 120.9, 117.4, 108.6, 47.4. HR-MS(ESI)m / z calcd for C 20 h 16 N 5 [M+H] + 326.1400,fou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com