Preparation method and application of polyvinyl alcohol hemostatic porous material with high liquid absorption and high expansion performance and active hemostatic function
A technology of polyvinyl alcohol and porous materials, applied in the field of medical devices, can solve the problems of complex removal of residual reagents, toxic and side effects of final products, lack of cell adhesion function and active hemostasis, etc. High expansion performance and strong liquid absorption ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
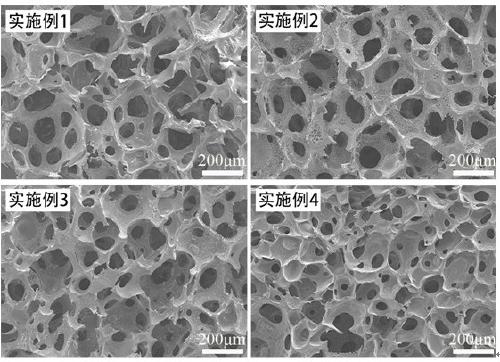
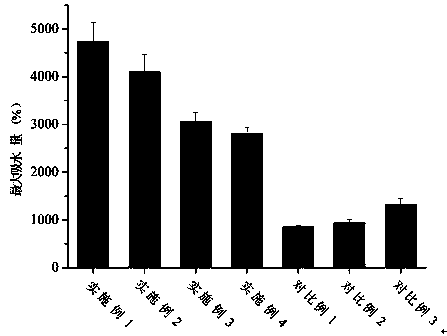
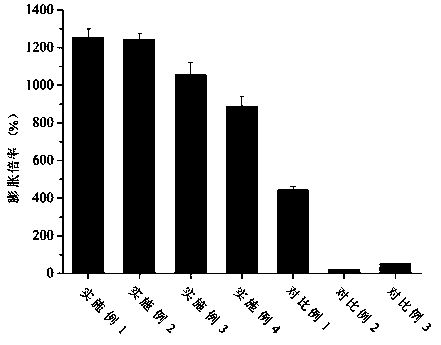
Embodiment 1
[0029] A preparation method of a polyvinyl alcohol hemostatic sponge with high liquid absorption and high swelling performance and active hemostatic function, comprising the steps of:
[0030] (1) Disperse polyvinyl alcohol 2099 into dimethyl sulfoxide to form a suspension with a mass fraction of 10%; heat to 100°C, stir for 2 hours, and cool to room temperature; Bornene diacid anhydride, continue to stir and react for 24 hours; place it in a dialysis bag with a molecular weight cut-off of 3500, dialyze for 3 days, and freeze-dry;
[0031] (2) Disperse the product obtained in step (1) in distilled water to form a solution with a mass fraction of 20%; add 0.5% of the product obtained in step (1) with photoinitiator 2959, 20% mercapto with a weight average molecular weight of 2000 - Polyethylene glycol-mercapto, 1% gelatin, 0.5% sodium lauryl sulfate, vigorously stirred at 1500rpm for 30min for foaming, and the foamed material was exposed to a light intensity of 60 μW / cm 2 UV l...
Embodiment 2
[0034] A preparation method of a polyvinyl alcohol hemostatic sponge with high liquid absorption and high swelling performance and active hemostatic function, comprising the following steps:
[0035] (1) Disperse polyvinyl alcohol 2699 in dimethyl sulfoxide to form a suspension with a mass fraction of 10%; heat to 100°C, stir for 2 hours, and cool to room temperature; Bornene diacid anhydride, continue to stir and react for 24 hours; place it in a dialysis bag with a molecular weight cut-off of 3500, dialyze for 3 days, and freeze-dry;
[0036] (2) Disperse the product obtained in step (1) in distilled water to form a solution with a mass fraction of 30%; add photoinitiator 2959 with a mass fraction of 0.7% of the product obtained in step (1), and 20% mercapto with a weight average molecular weight of 2000 - Polyethylene glycol-mercapto, 1% gelatin, 0.5% sodium lauryl sulfate, vigorously stirred at 1500rpm for 30min for foaming, and the foamed material was exposed to light at ...
Embodiment 3
[0039] A preparation method of a polyvinyl alcohol hemostatic sponge with high liquid absorption and high swelling performance and active hemostatic function, comprising the following steps:
[0040](1) Disperse polyvinyl alcohol 2699 into dimethyl sulfoxide to form a suspension with a mass fraction of 20%; heat to 100°C, stir for 2 hours, and cool to room temperature; Bornene diacid anhydride, continue to stir and react for 24 hours; place it in a dialysis bag with a molecular weight cut-off of 3500, dialyze for 3 days, and freeze-dry;
[0041] (2) Disperse the product obtained in step (1) in distilled water to form a solution with a mass fraction of 30%; add 0.5% photoinitiator 2959 of the product obtained in step (1), 20% mercapto with a weight average molecular weight of 2000 - Polyethylene glycol-mercapto, 5% gelatin, 0.5% sodium lauryl sulfate, vigorously stirred at 1500rpm for 30min for foaming, and the foamed material was exposed to light at a light intensity of 60 μW / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com