Construction of photosensitizer-hypoxia activated prodrug integrated prodrug self-assembled nanoparticles
A technology of self-assembled nanoparticles and photosensitizers, which can be used in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of aggregation quenching effect, leakage of photosensitizers, low drug loading, etc., to prolong the cycle time and prepare The effect of simple process and easy surface modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
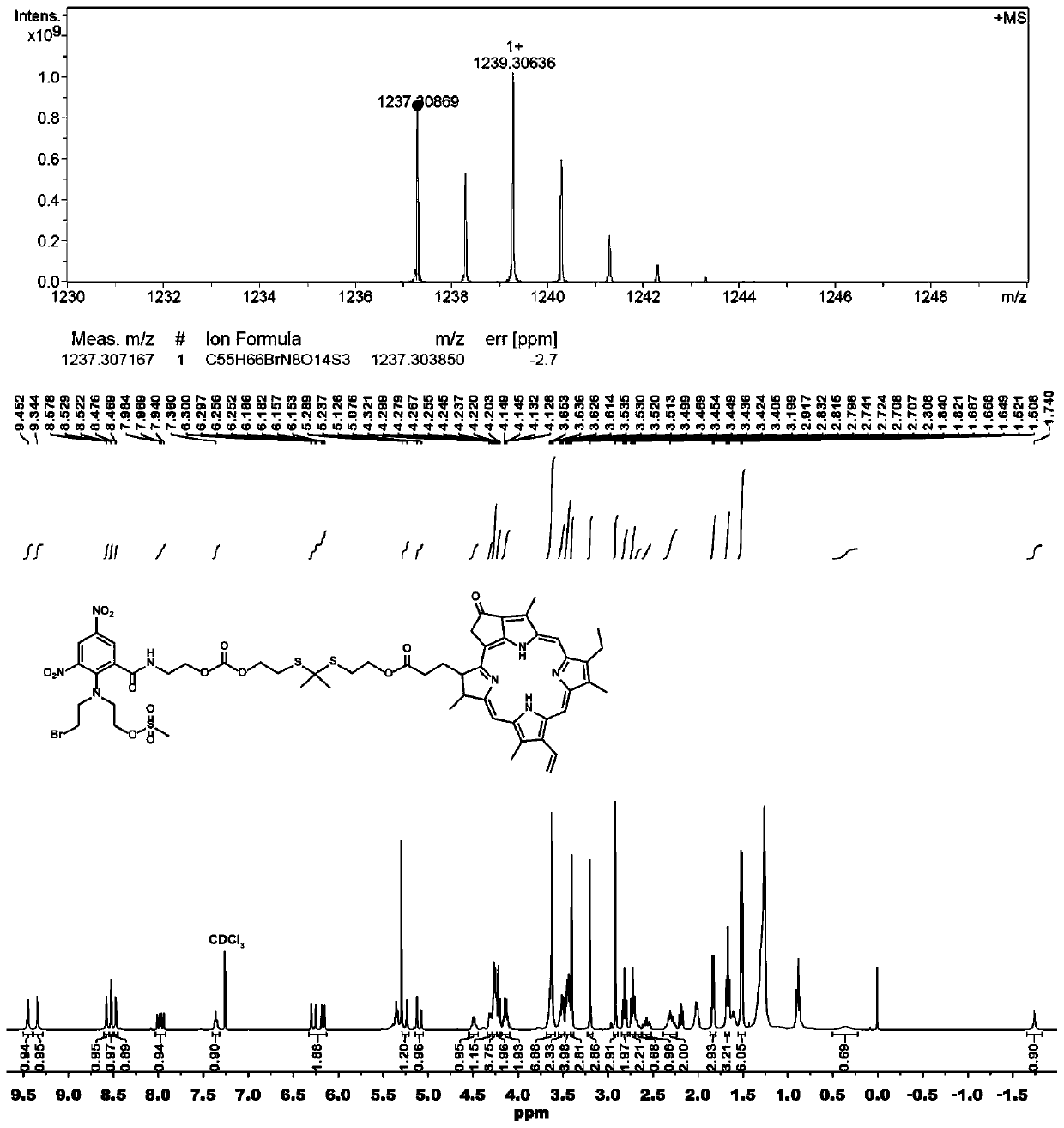
[0045] Synthesis of PR104A-pyropheophorbide a prodrug (PR104A-TK-PPa) linked by thioketal bond
[0046] Dissolve PR104A and triphosgene in anhydrous dichloromethane and incubate in a low-temperature ice bath, dissolve DMAP in a small amount of dichloromethane and add it dropwise to the above mixed solution, and react under ice bath for 1-2 hours. Add 4,4-dimethyl-3,5-dithioheptanediol into the reaction liquid, react at room temperature for 24 hours, and separate and purify to obtain an intermediate product. In addition, PPa, N-methylmorpholine and HATU were dissolved in anhydrous dichloromethane and incubated in a low-temperature ice bath, and reacted in an ice bath for 1-2 hours. The above-mentioned intermediate product was added to the reaction liquid, reacted at room temperature for 24 hours, and PR104A-TK-PPa was obtained by separation and purification.
[0047] Adopt mass spectrometry and proton nuclear magnetic resonance spectrometry to determine the structure of the pr...
Embodiment 2
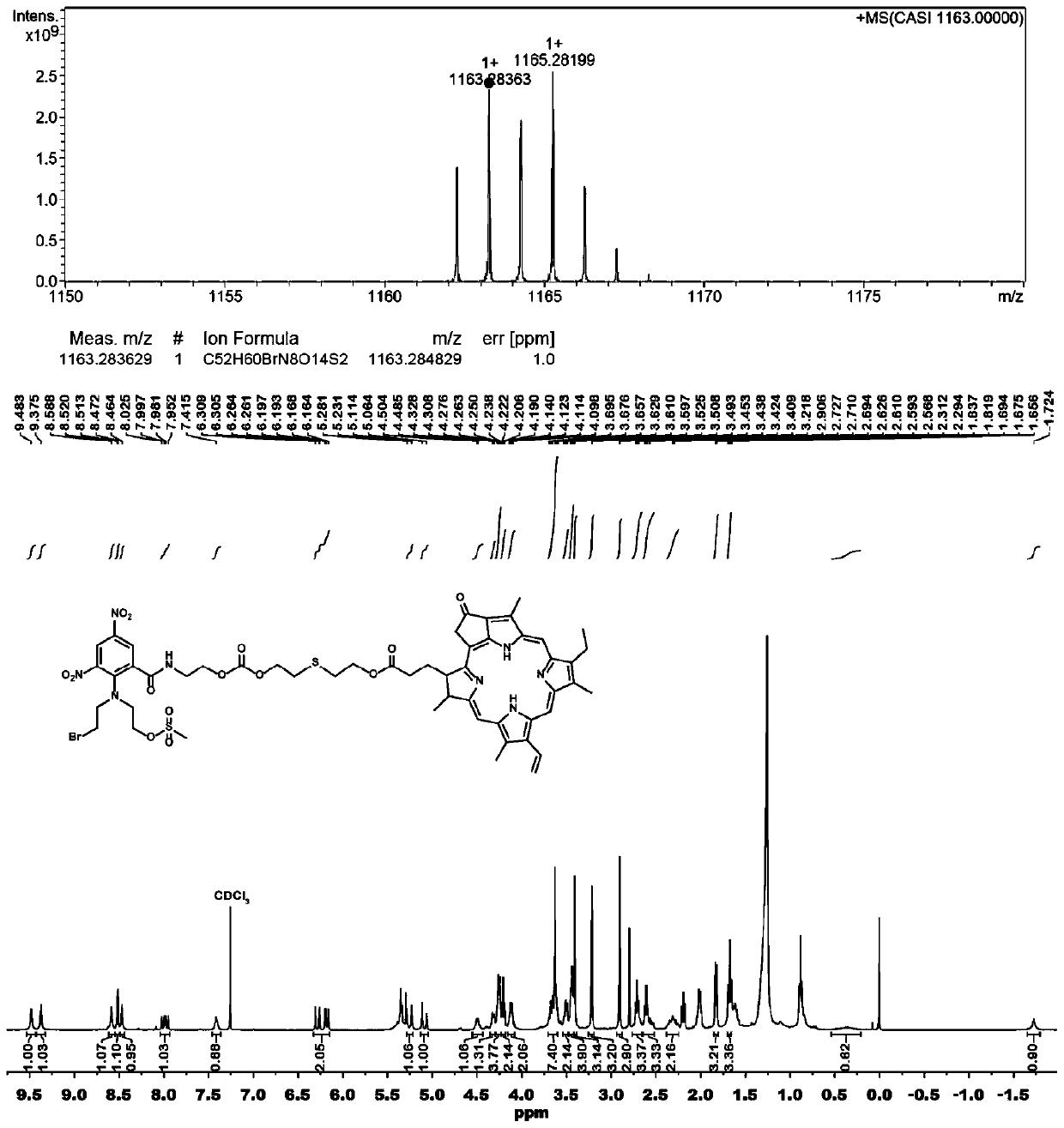
[0050] Synthesis of PR104A-pyropheophorbide a prodrug (PR104A-S-PPa) linked by monosulfide bond
[0051] Dissolve PR104A and triphosgene in anhydrous dichloromethane and incubate in a low-temperature ice bath, dissolve DMAP in a small amount of dichloromethane and add it dropwise to the above mixed solution, and react under ice bath for 1-2 hours. Add thiodiethanol to the reaction solution, react at room temperature for 24 hours, and separate and purify to obtain an intermediate product. In addition, PPa, N-methylmorpholine and HATU were dissolved in anhydrous dichloromethane and incubated in a low-temperature ice bath, and reacted in an ice bath for 1-2 hours. The above-mentioned intermediate product was added to the reaction liquid, reacted at room temperature for 24 hours, and PR104A-S-PPa was obtained by separation and purification.
[0052] Adopt mass spectrometry and proton nuclear magnetic resonance spectrometry to determine the structure of the prodrug in embodiment 2...
Embodiment 3
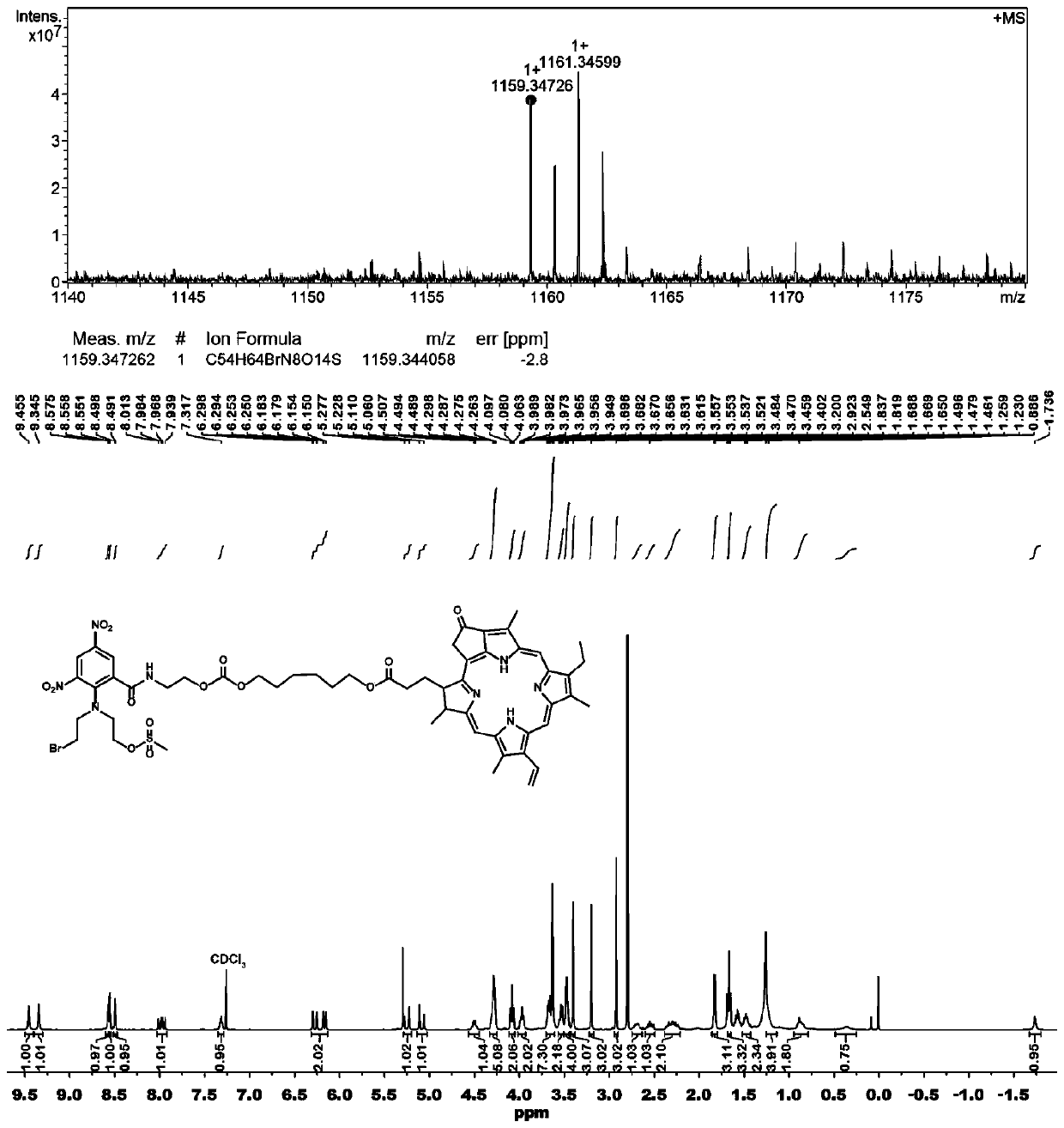
[0055] Synthesis of PR104A-pyropheophorbide a prodrug (PR104A-PPa) linked by non-sensitive carbon chain
[0056] Dissolve PR104A and triphosgene in anhydrous dichloromethane and incubate in a low-temperature ice bath, dissolve DMAP in a small amount of dichloromethane and add it dropwise to the above mixed solution, and react under ice bath for 1-2 hours. 1,6-Hexanediol was added to the reaction solution, reacted at room temperature for 24 hours, and the intermediate product was obtained by separation and purification. In addition, PPa, N-methylmorpholine and HATU were dissolved in anhydrous dichloromethane and incubated in a low-temperature ice bath, and reacted in an ice bath for 1-2 hours. The above-mentioned intermediate product was added to the reaction solution, reacted at room temperature for 24 hours, and was separated and purified to obtain PR104A-PPa.
[0057] Adopt mass spectrometry and proton nuclear magnetic resonance spectrometry to determine the structure of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com