Carboxylated polycaprolactone-based 5-aminolevulinic acid methyl ester prodrug and preparation method and application thereof
A technology of methyl aminolevulinate and polycaprolactone, which can be used in pharmaceutical formulations, antineoplastic drugs, drug combinations, etc., can solve the problems of poor fat solubility and instability, and achieve high conversion rate and good photodynamic efficacy , good lethal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
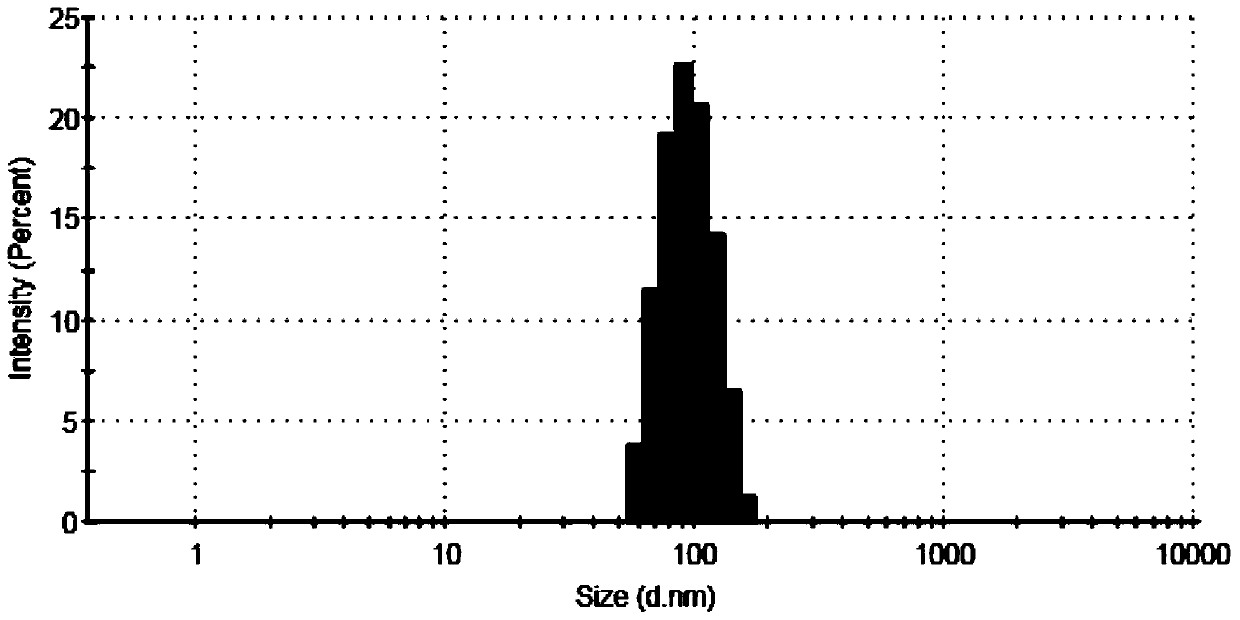
Examples
Embodiment 1
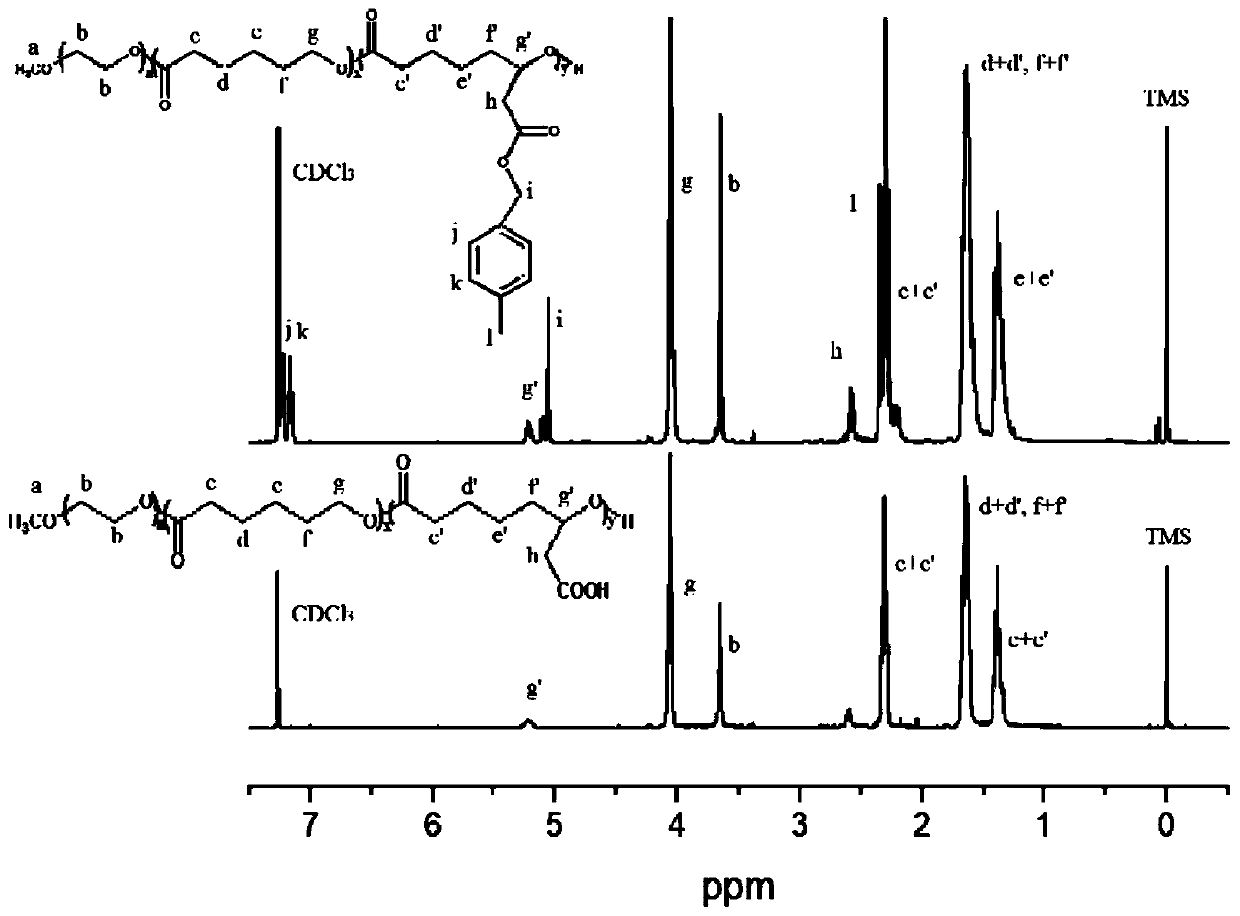
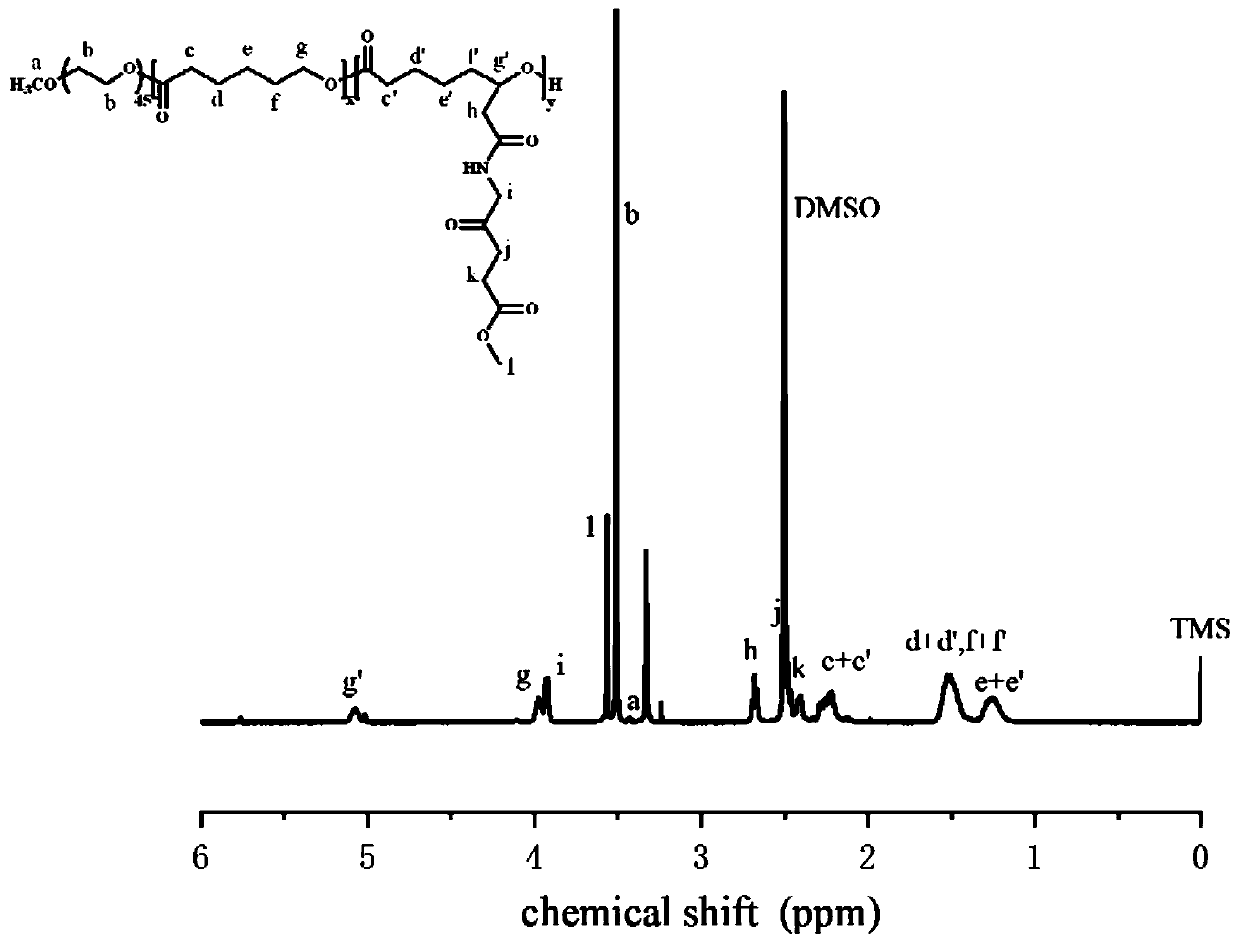
[0035] Synthesis of mPEG-b-P(CL-co-BCL) and mPEG-b-P(CL-co-CCL)
[0036]
[0037] x=30, y=20.
[0038] Under argon protection, 1 g of polyethylene glycol monomethyl ether (mPEG 2k ), 2.79g 6(2)-p-methylbenzyl acetate-ε-caprolactone (6(2)-p-(CH 3 ) BCL) and 1.71g ε-caprolactone (ε-CL) were added to the dry polymerization bottle, and the polymerization bottle was placed in an oil bath at a temperature of 60°C, continuously stirred and vacuumed for 2 hours, and then 50 microliters of iso Stannous octoate (Sn(Oct) 2 ) (10mg, 0.025mmol) of the toluene solution was added to the polymerization bottle, through repeated vacuuming-argon gas operation, to remove a small amount of toluene solvent, then filled with argon in the polymerization bottle, sealed and placed in the oil at a temperature of 130 ° C The reaction was continued in the bath for 24 hours. After the reaction was completed, it was dissolved with a small amount of DCM, and then a large amount of ice anhydrous ether w...
Embodiment 2
[0047] Synthesis of mPEG-b-P(CL-co-BCL) and mPEG-b-P(CL-co-CCL)
[0048]
[0049] x=40, y=10.
[0050] Under argon protection, 1 g of polyethylene glycol monomethyl ether (mPEG 2k ), 1.395g 6(2)-p-methylbenzyl acetate-ε-caprolactone (6(2)-p-(CH 3 ) BCL) and 2.28g ε-caprolactone (ε-CL) were added to the dry polymerization bottle, and the polymerization bottle was placed in an oil bath at a temperature of 60°C, kept stirring and vacuuming for 2 hours, and then 50 microliters of iso Stannous octoate (Sn(Oct) 2 ) (10mg, 0.025mmol) of the toluene solution was added to the polymerization bottle, through repeated vacuuming-argon gas operation, to remove a small amount of toluene solvent, then filled with argon in the polymerization bottle, sealed and placed in the oil at a temperature of 130 ° C The reaction was continued in the bath for 24 hours. After the reaction was completed, it was dissolved with a small amount of DCM, and then a large amount of ice anhydrous ether was ad...
Embodiment 3
[0059] Synthesis of mPEG-b-P(CL-co-BCL) and mPEG-b-P(CL-co-CCL)
[0060]
[0061] x=20, y=30.
[0062] Under argon protection, 0.5 g polyethylene glycol monomethyl ether (mPEG 2k ), 2.093g 6(2)-p-methylbenzyl acetate-ε-caprolactone (6(2)-p-(CH 3 ) BCL) and 0.57g ε-caprolactone (ε-CL) were added to the dry polymerization bottle, and the polymerization bottle was placed in an oil bath at a temperature of 60°C, and kept stirring and vacuuming for 2 hours, and then 25 microliters of iso Stannous octoate (Sn(Oct) 2 ) (5mg, 0.0125mmol) of toluene solution was added to the polymerization bottle, through repeated vacuuming-argon gas operation, to remove a small amount of toluene solvent, then filled with argon in the polymerization bottle, sealed and placed in the oil at a temperature of 130 ° C The reaction was continued in the bath for 24 hours. After the reaction was completed, it was dissolved with a small amount of DCM, and then a large amount of ice anhydrous ether was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com