Preparation and application of cryptosporidium protein kinase 660 C-terminal protein
A protein kinase and cryptosporidium technology, which is applied in the field of in vitro recombinant protein preparation, can solve the problems of inability to express, self-cleavage and degradation, and undetermined functions, and achieves the effects of improving solubility, increasing yield and protecting integrity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A method for preparing a cryptosporidium interspecies and intraspecies highly polymorphic protease 660 C-terminal protein, comprising the steps of:
[0045] 1. Construction of Cp660C protein recombinant expression system
[0046] Using Cryptosporidium genomic DNA as a template, design specific amplification primers (SEQ ID NO: 1-2),
[0047] F: 5'-CGCGGATCCAAGACTGGAGATTTGA-3' (SEQ ID NO: 1);
[0048] R: 5'-CCGCTCGAGTTTAGGTGGAGGAGGT-3' (SEQ ID NO: 2);
[0049] The PCR amplification reaction system is: Phusion enzyme 0.5 μL, upstream primer 2.5 μL, downstream primer 2.5 μL, DNA template 1 μL, 2mM dNTPs 5 μL, 5x Phusion HF buffer 10 μL, ddH 2 O 28.5 μL, a total of 50 μL.
[0050] The PCR amplification reaction program was: pre-denaturation at 98°C for 5 min; denaturation at 98°C for 45 s, annealing at 58°C for 45 s, extension at 72°C for 35 s, and 35 cycles; final extension at 72°C for 7 min.
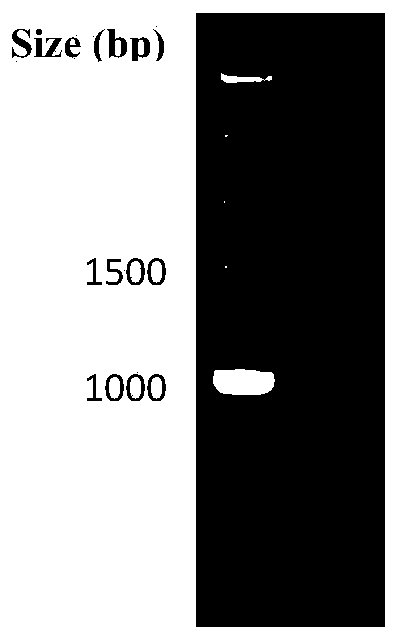
[0051] The Cp660C gene fragment (SEQ ID NO: 3) with a fragment length of 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com