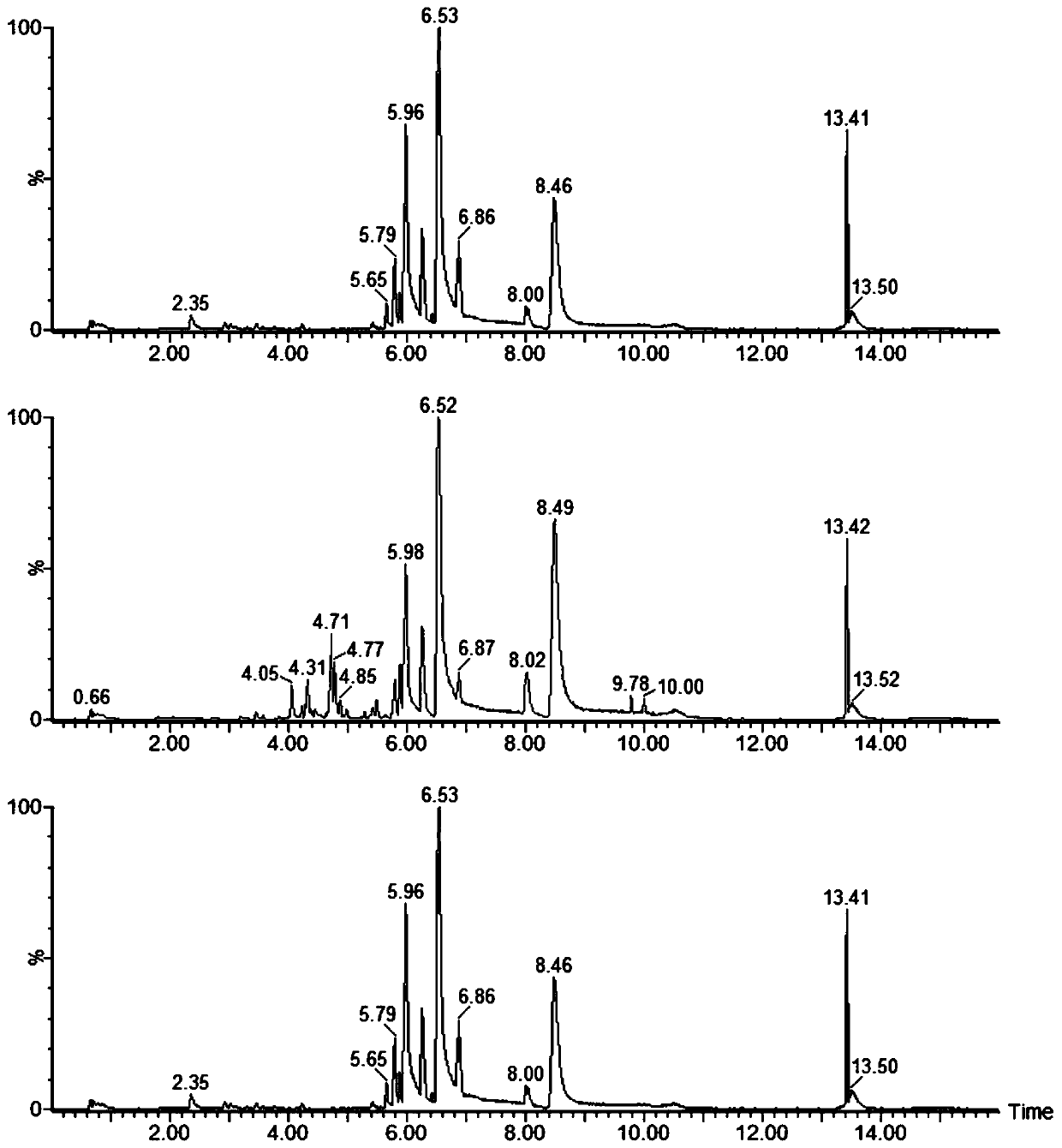
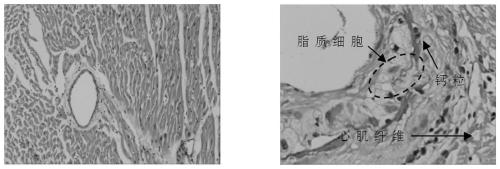
Composition for treating coronary heart disease
A composition and technology for coronary heart disease, applied in the fields of medicine and pharmacy, can solve the problems of large dosage, unclear mechanism of action, unclear metabolism in the body, etc., and achieve the effect of simple composition and treatment of coronary heart disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 a composition
[0062] The composition described in this embodiment consists of the following components in parts by weight (1 part by weight=100g):
[0063] 5 parts by weight of puerarin D, 15 parts by weight of 3'-methoxydaidzin, 5 parts by weight of formononetin, 30 parts by weight of 3'-methoxypuerarin, 25 parts by weight of puerarin, 3'-hydroxypuerarin 30 parts by weight of ginseng, 1.0 parts by weight of maslinic acid, 1.0 parts by weight of hyperoside, 2.0 parts by weight of gypenoside A, 1.5 parts by weight of gypenoside LXXV and 2.0 parts by weight of gypenoside XLIX.
[0064] The above components are uniformly mixed in parts by weight to obtain the target composition.
Embodiment 2
[0065] Example 2 a composition
[0066] The composition described in this embodiment consists of the following components in parts by weight (1 part by weight=100g):
[0067] 2 parts by weight of puerarin D, 7 parts by weight of 3'-methoxydaidzein, 2 parts by weight of formononetin, 15 parts by weight of 3'-methoxypuerarin, 42 parts by weight of puerarin, 3'-hydroxypuerarin 15 parts by weight of quinine, 0.8 parts by weight of maslinic acid, 0.8 parts by weight of hyperoside, 1.8 parts by weight of gypenoside A, 1.2 parts by weight of gypenoside LXXV and 1.8 parts by weight of gypenoside XLIX.
[0068] The above components are uniformly mixed in parts by weight to obtain the target composition.
Embodiment 3
[0069] Example 3 a composition
[0070] The composition described in this embodiment consists of the following components in parts by weight (1 part by weight=100g):
[0071] 3.5 parts by weight of puerarin D, 8.5 parts by weight of 3'-methoxydaidzin, 3.5 parts by weight of formononetin, 18 parts by weight of 3'-methoxypuerarin, 38 parts by weight of puerarin, 3'-hydroxypuerarin 22 parts by weight of ginseng, 0.5 parts by weight of maslinic acid, 0.5 parts by weight of hyperoside, 1.4 parts by weight of gypenoside A, 1.1 parts by weight of gypenoside LXXV and 1.7 parts by weight of gypenoside XLIX.
[0072] The above components are uniformly mixed in parts by weight to obtain the target composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com