Synthetic method of methyl ketone compound
A synthesis method and technology for methyl ketones are applied in the field of synthesis of methyl ketone compounds, can solve problems such as application limitations, and achieve the effects of simple operation, high conversion rate and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
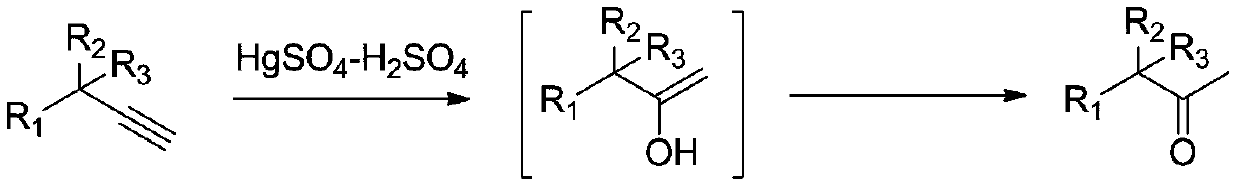
[0027] The invention provides a kind of synthetic method of methyl ketone compound, comprises the following steps:
[0028] Mixing a terminal alkyne having a structure shown in formula I, an organic solvent, an acid and water for a hydration reaction to obtain a methyl ketone compound;
[0029]
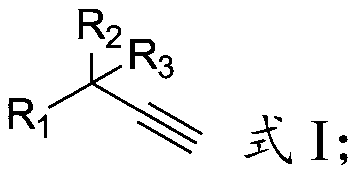
[0030] In formula I: R 1 is hydrocarbyl, substituted hydrocarbyl, hydrocarbyl formyl, aryl, aryl formyl, substituted aryl, substituted aryl formyl, heterocycle or heterocyclic formyl, R 2 and R 3 are independently hydrocarbyl, substituted hydrocarbyl or cyclohydrocarbyl.
[0031] The present invention has no special requirements on the source of the terminal alkyne having the structure shown in formula I, and it can be prepared by using commercially available products or by methods well known to those skilled in the art.
[0032] The reaction equation of the present invention's synthetic methyl ketone compound is shown in formula a:
[0033]
[0034] In the present invention...
Embodiment 1
[0047] Embodiment 1: the synthesis of 3-phenyl-3-methylbutan-2-one
[0048] Reaction formula:
[0049]
[0050] Reaction raw materials and consumption are shown in Table 1:
[0051] Table 1 Reaction raw materials and consumption
[0052]
[0053] Dissolve 144.2 g of (2-methylbutyl-3-yn-2-yl)benzene in 720 g of tetrahydrofuran, add 110.6 g of 33 wt% hydrochloric acid solution and 21.6 g of water, raise the temperature to 40° C., and stir for 6 hours. After all the raw materials were converted, the reaction mixture was cooled to room temperature, washed with 500 mL of aqueous sodium bicarbonate solution and 500 mL of brine, separated the organic phase, and concentrated under reduced pressure to remove the solvent. The obtained residue was distilled under reduced pressure to obtain pure 3-phenyl-3-methyl-butan-2-one.
[0054] 156.2 g (0.96 mol) of the product were obtained, yield: 96.4%, gas chromatography purity: 99.2%.
Embodiment 2
[0055]Example 2: Synthesis of 3-cyclohexyl-3-methylbutan-2-one
[0056] Reaction formula:
[0057]
[0058] The reaction raw materials and consumption are shown in Table 2:
[0059] Table 2 Reaction raw materials and consumption
[0060]
[0061]
[0062] Dissolve 150.1 g of (2-methylbutyl-3-yn-2-yl)cyclohexane in 720 g of tetrahydrofuran, add 98 g of 50 wt% sulfuric acid solution and 21.6 g of water, raise the temperature to 30°C, and stir for 7.5 hours , after all the raw materials were converted, the reaction mixture was cooled to room temperature, washed with 500 mL of aqueous sodium bicarbonate solution and 500 mL of brine, separated the organic phase, and concentrated under reduced pressure to remove the solvent. The obtained residue was distilled under reduced pressure to obtain pure 3-cyclohexyl-3-methylbutan-2-one.
[0063] 160 g (0.95 mol) of the product were obtained, the yield: 95%, and the purity by gas chromatography: 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com