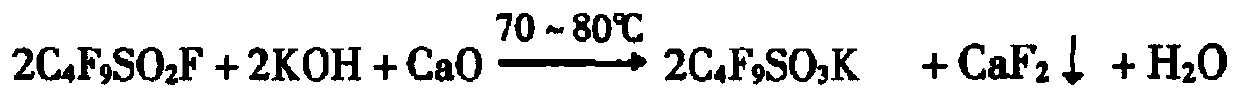Preparation method of potassium perfluorobutanesulfonate
A technology of potassium perfluorobutanesulfonate and perfluorobutanesulfonyl fluoride is applied in the field of continuous reaction preparation of potassium perfluorobutanesulfonate, which can solve the influence of by-product calcium fluoride on the purity, low production efficiency and easy leakage And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The molar ratio of the reaction solution is set as perfluorobutylsulfonyl fluoride: potassium hydroxide: calcium hydroxide: water as 1:1.1:0.55:50. The aqueous solution of potassium hydroxide and calcium hydroxide is mixed in proportion to make material A; Tubular reactor (reaction tube length 10 meters, pipe diameter φ 8, water bath, purchased from Shanghai Sanaifu New Material Technology Co., Ltd.) is heated to 80°C, after stabilization, inject material A and perfluorobutylsulfonyl fluoride into the tubular reactor at the same time according to the molar ratio set above, and control the reactor pressure at 0.25MPa by controlling the outlet flow rate, so that the perfluorobutanesulfonyl fluoride Butylsulfonyl fluoride remains in a liquid state, and the slurry containing potassium perfluorobutanesulfonate and calcium fluoride generated by the reaction is directly sprayed into the drying device by using the pressure of the reaction tube (the oven temperature is 120°C-160°...
Embodiment 2
[0056] Referring to Example 1, other conditions remain unchanged, and the reaction temperature is adjusted to 85°C.
Embodiment 3
[0058] Referring to Example 1, other conditions remain unchanged, and the reaction temperature is adjusted to 95°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com