A method of using ion transporter to promote the synthesis of L-arginine by Corynebacterium bacillus
A technology of Corynebacterium blunt tooth and antiporter, which is applied in the field of bioengineering to achieve the effect of increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
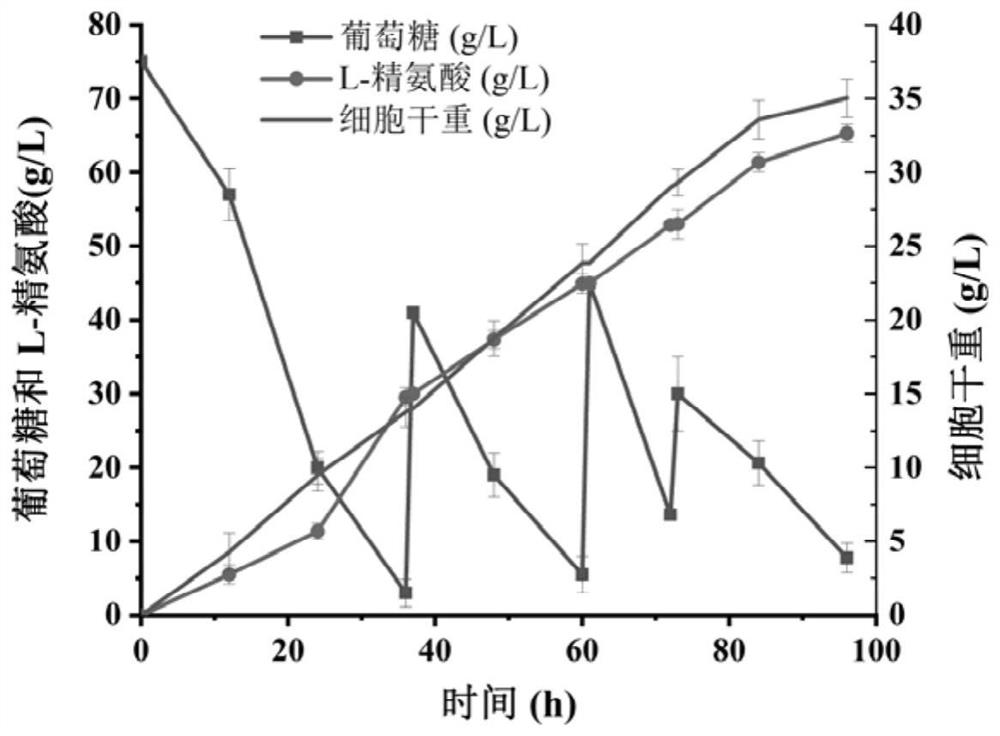
Image
Examples
Embodiment 1
[0025] Example 1: Knockout of ion transporters Mrp1A and CTAP1
[0026] With Corynebacterium bacillus SYPA5-5 genome as template, utilize primer pair Δmrp1-1 / Δmrp1-2 (nucleotide sequence as shown in SEQ ID NO.5 / 6) and Δmrp1-3 / Δmrp1-4 (nucleotide The sequence is shown in SEQ ID NO.7 / 8) to amplify the upstream and downstream fragments of the mrp1 gene by 500 bp each. After recovery, use the above and downstream fragments as templates, and use mrp1-1 (nucleotide sequence as shown in SEQ ID NO.5) and mrp1-4 (nucleotide sequence as shown in SEQ ID NO.8) primer fusion PCR amplification The missing fragment of the mrp1 gene was obtained. Purify and recover the PCR product to obtain the mrp1 gene deletion fragment, purify and recover the digested product of pK18mobSacB plasmid, and use homologous recombinase for connection. The ligation product was transformed into E.coli JM109 competent by heat shock, and the transformants were selected by culture on LB plates containing kanamycin ...
Embodiment 2
[0029] Example 2: Overexpression of the ion transporters Mrp1A and CTAP1
[0030] With Corynebacterium bacillus SYPA5-5 genome as template, utilize primer pair 10-mrp1ctap1-1 / 2 (nucleotide sequence as shown in SEQ ID NO.13 / 14) and 10-mrp1ctap1-3 / 4 (nucleotide The sequence is shown in SEQ ID NO.15 / 16) to amplify the upstream mrp1 and downstream ctap1 gene fragments respectively. After recovery, the above and downstream fragments were used as templates, and the tandem gene mrp1ctap1 was amplified by fusion PCR using the primer pair 10-mrp1ctap1-1 / 4. Purify and recover the PCR product to obtain the mrp1ctap1 gene fragment, purify and recover the digested product of the pDXW-10 plasmid, and use homologous recombinase for connection. The ligation product was transformed into E.coli BL21 competent by heat shock, and the transformants were screened by kanamycin resistance plate culture, and the plasmid was extracted and digested, PCR and sequencing were verified. The verification w...
Embodiment 3
[0031] Example 3: Mrp1A and CTAP1 on intracellular [Na + ] and [K + ]Impact
[0032] Take 1mL of bacterial liquid, centrifuge at 8000r / min for 2min, discard the supernatant, wash the bacterial cells with distilled water 3 times, dry at 60°C, weigh the dry cell weight, 1OD 562 =0.375g / L dry weight of cells. Take 1mL of bacteria, digest completely with 1mL of concentrated nitric acid until clarified, and then dilute 100 times. The diluted liquid was filtered through a membrane, and the recombinant strain [Na + ] / [K + ] content. Calibrate with ultrapure water.
[0033] At pH 7.0, the Na of the 5-5Δmrp1Δctap1 mutant compared + concentration increased significantly, while K + The concentration is lower. This is due to the absence of ion transporters leading to intracellular Na + cannot be transported out of the cell, intracellular Na + Accumulation leads to a significant increase in concentration, and the cell needs to adjust the intracellular ionic strength, so that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com