Application of faropenem in preparation of medicine for initial treatment of pathogen-positive pulmonary tuberculosis
A faropenem and pulmonary tuberculosis technology, applied in the field of medicine, can solve the problems of high toxicity and side effects, long course of treatment, etc., and achieve the effect of high cure rate, fast onset of effect, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Determination of MIC of each drug on Mycobacterium tuberculosis
[0034] The human tuberculosis standard strain H37Rv (purchased from Shandong Institute of Tuberculosis Prevention and Treatment, batch number: 170308) was inoculated into Roche slant medium and cultured at 37°C for 4 weeks. Centrifuge the appropriate amount of culture and mix it with physiological saline. After 25 minutes of precipitation, take the upper bacterial solution and place it on the Roche slant medium containing different concentrations of drugs. The inoculum concentration is 10 6 CFU / point, cultivate at 37°C until colonies are formed in the growth control tube, observe and record the results.
[0035] The preparation method of the drug-containing Roche slant medium is the same as that of the ordinary Roche slant medium, and the medicine is added to the medium before the medium is added to the test tube.
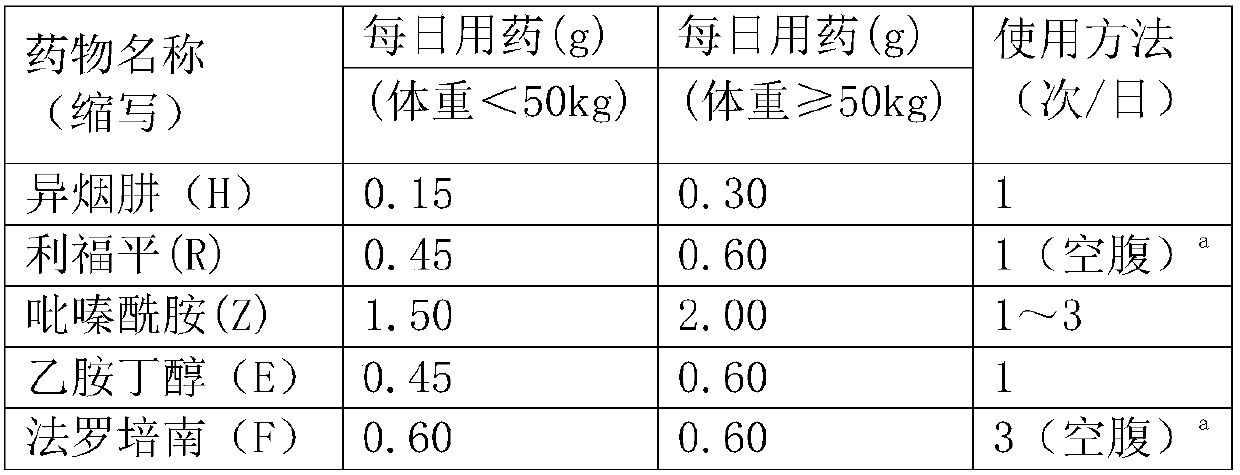
[0036] The drugs used in the experimental group were: Faropenem, Pyrazinamide and Rifa ...
Embodiment 2
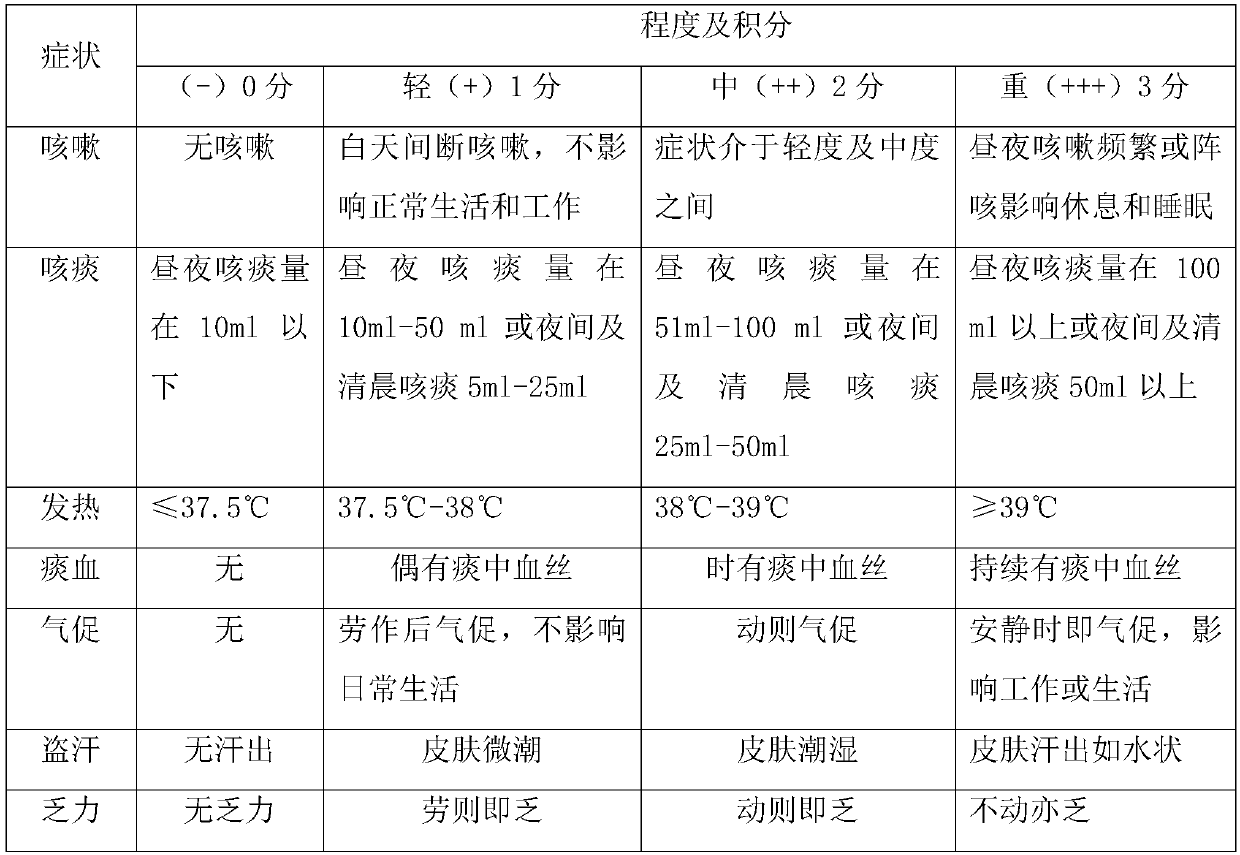
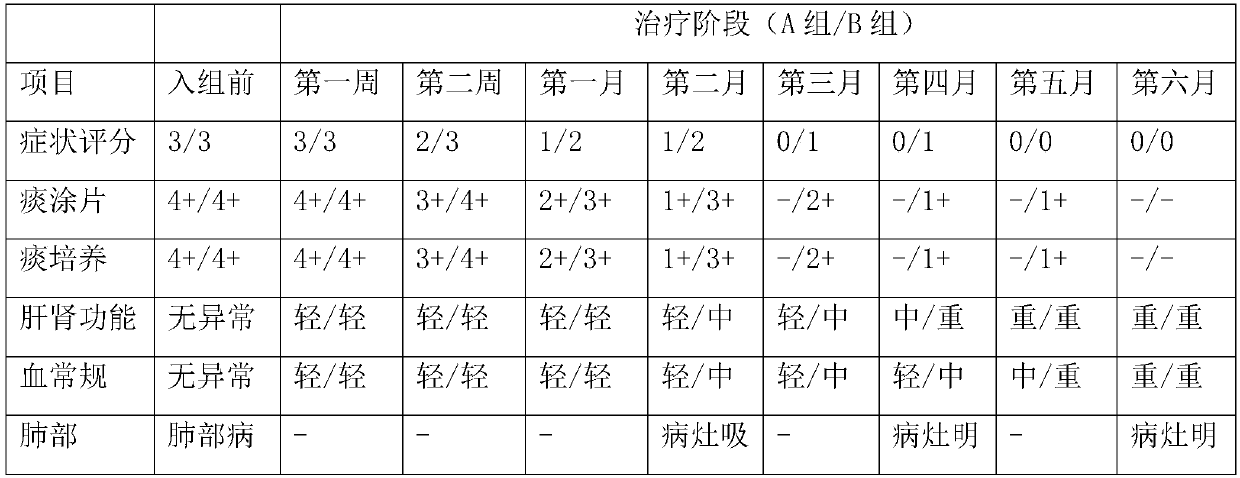
[0042] Example 2 Clinical trial to investigate the therapeutic effect of faropenem on newly diagnosed pathogen-positive pulmonary tuberculosis
[0043] 1. Case inclusion
[0044] (1) Case inclusion criteria
[0045] 1) Patients diagnosed with tuberculosis for the first time with positive pathogen identification, including sputum smear-positive, sputum culture-positive, GeneXpert-positive, among them, sputum smear-positive, sputum culture-positive patients are identified as Mycobacterium tuberculosis.
[0046] 2) Age 18 to 80 years old.
[0047] 3) The susceptibility of sputum bacteria confirmed that there is no resistance to isoniazid and rifampicin.
[0048] 4) The liver and kidney functions are normal.
[0049] 5) Imaging shows lung tuberculosis lesions.
[0050] 6) No HIV infection.
[0051] (2) Case exclusion criteria:
[0052] 1) Pulmonary tuberculosis combined with extrapulmonary tuberculosis.
[0053] 2) A history of allergies to any drugs in the protocol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com