Crystal form of flomoxef sodium, preparation method, pharmaceutical composition and application
A technology of sodium fluoxefaclor and a composition, applied in the field of drug crystals, can solve the problems of inability to be removed by drying, no clear disclosure of the crystal form of sodium fluoxefate, and organic solvents affecting medicinal value, etc., and achieve the advantage of good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
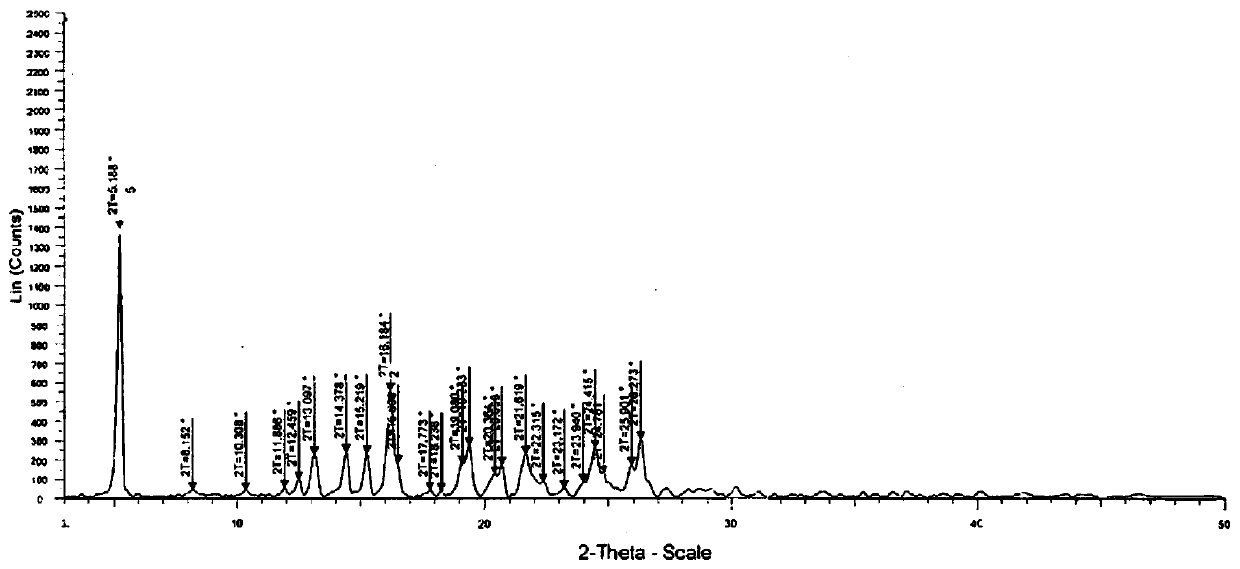
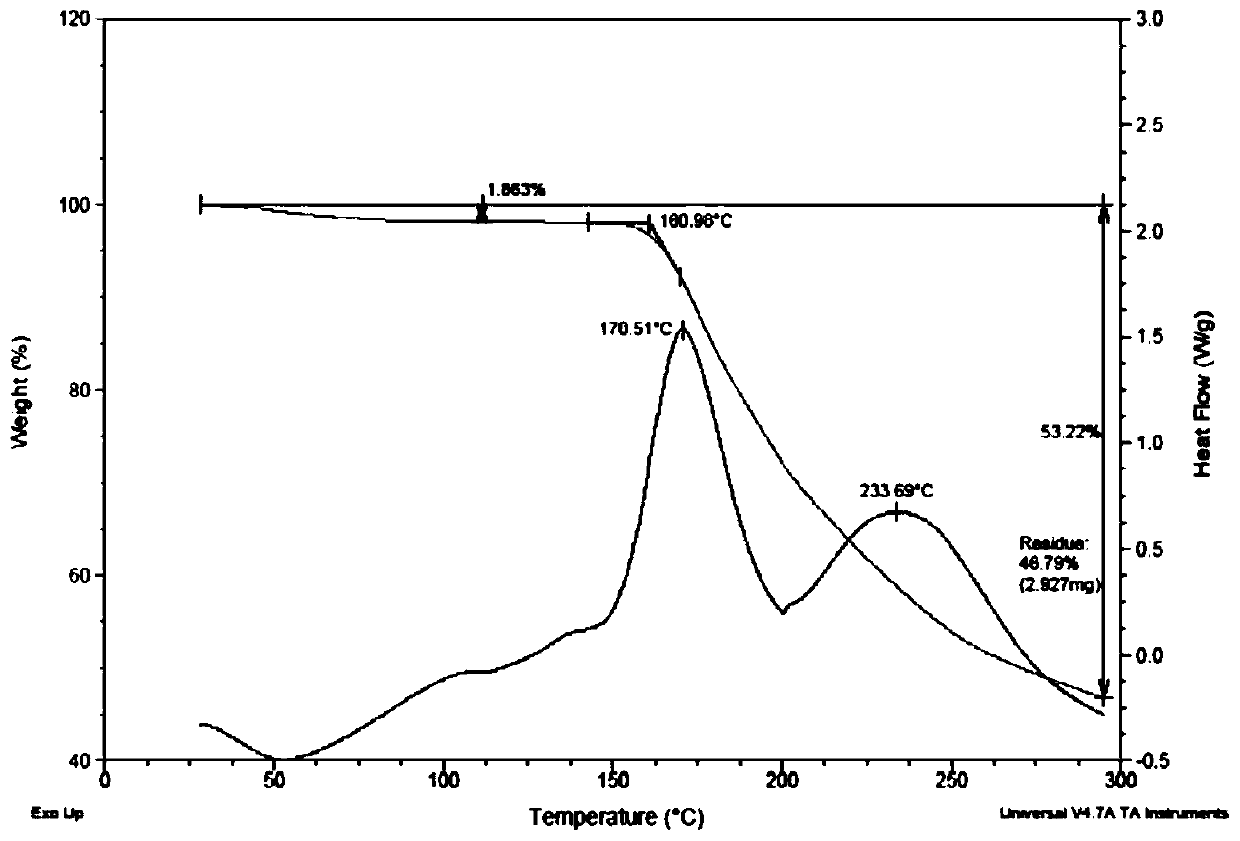
Image
Examples
Embodiment 1
[0051] Add 40 grams of fluoxetal acid to a mixed solvent of 400 milliliters of ethylene glycol and 400 milliliters of isobutanol, and stir to dissolve. 5% (W / W) sodium bicarbonate aqueous solution was added dropwise under stirring to adjust the pH to 4.5-6.0. Afterwards, stirring and cooling down to -10°C, continued stirring and crystallization for 5 hours, the suspension was filtered, and the obtained crystals were washed with isobutanol, and vacuum-dried at 25°C to constant weight to obtain 26.6 grams of white granular crystals of fluoxetal sodium, The purity of the crystal form obtained in this example was determined to be 98.4% by high performance liquid chromatography (HPLC).
Embodiment 2
[0053] Add 20 grams of fluoxetal acid to a mixed solvent of 150 milliliters of ethylene glycol and 150 milliliters of isobutanol, and stir to dissolve. Add about 8% (W / W) sodium bicarbonate aqueous solution dropwise under stirring to adjust the pH to 4.5-6.0. Then cool down to -15°C under stirring, continue to stir and crystallize for 5 hours, filter the suspension, wash the obtained crystals with isobutanol, and vacuum-dry at 25°C to constant weight to obtain 15.8 grams of white granular crystals of fluoxetal sodium , the purity of the crystalline form obtained in this example was measured by high performance liquid chromatography (HPLC) to be 99.1%.
Embodiment 3
[0055] Add 50 grams of fluoxetal acid to a mixed solvent formed by 375 milliliters of ethylene glycol and 375 milliliters of isobutanol, and stir to dissolve. About 10% (W / W) sodium bicarbonate aqueous solution (saturated solution stirred for 1 hour at 25° C.) was added dropwise with stirring to adjust the pH to 4.5-6.0. Then cool down to -20°C under stirring, continue to stir and crystallize for 5 hours, filter the suspension, wash the obtained crystals with isobutanol, and vacuum-dry at 25°C to constant weight to obtain 40.1 g of white granular crystals of fluoxetal sodium , the purity of the crystalline form obtained in this example was measured by high performance liquid chromatography (HPLC) to be 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com