Visual field clearing agent for endoscope
A technology of endoscope and field of view, applied in the field of medical endoscope, to achieve the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
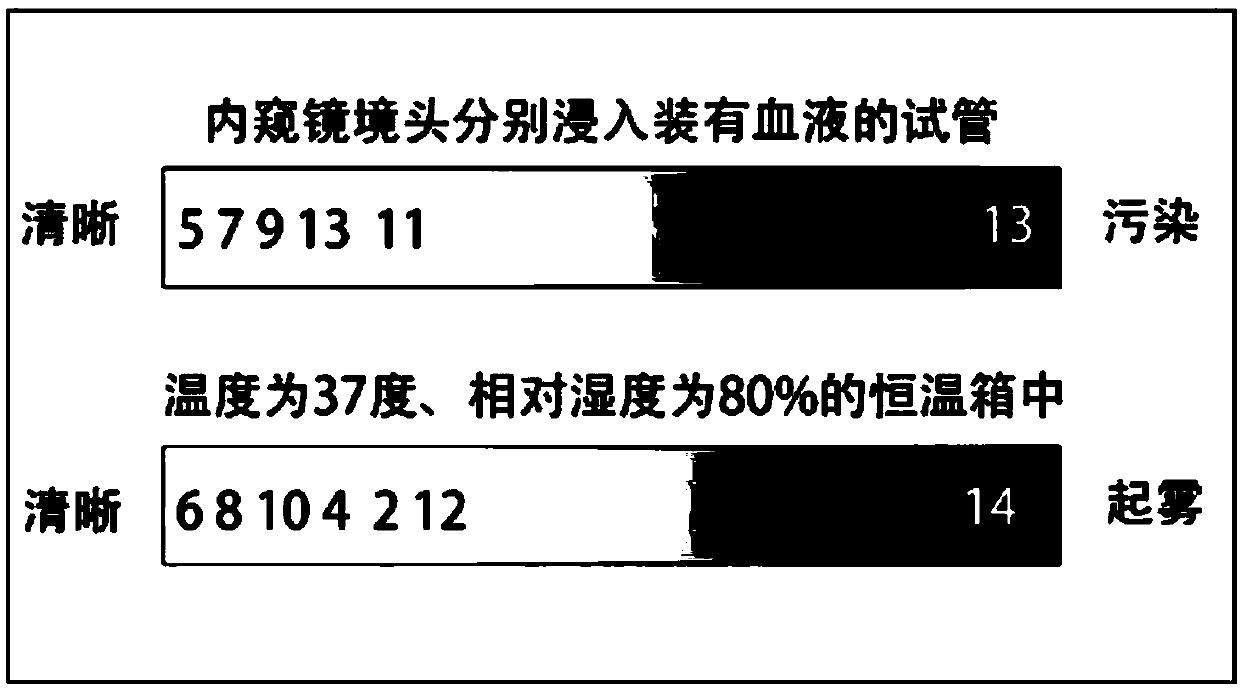
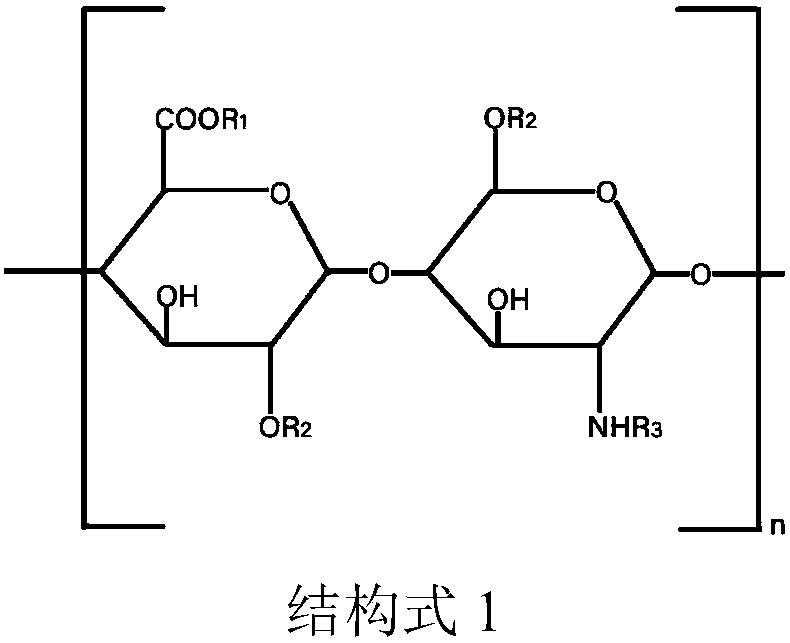
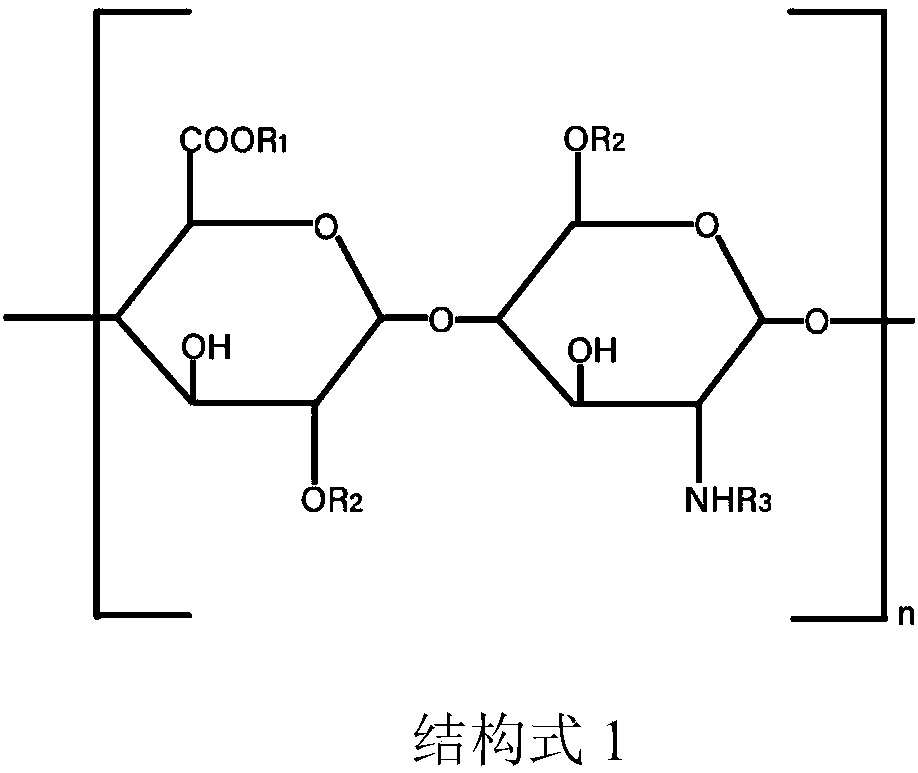
[0046] In medical sodium chloride aqueous solution 100mml, add the sodium salt injection of 0.01mg aminodextran sulfate (sodium heparin, Changzhou Qianhong Biochemical Pharmaceutical Co., Ltd., molecular weight is 15 000-19 000 Daltons, in structural formula 1, n=25-32), and 0.15 mg of sodium chloride, to prepare endoscope vision clearing agent 1 (the content of mucopolyglycolipid is about 0.01%, and the content of sodium chloride is about 1.5%).
Embodiment 2
[0048] In medical sodium chloride aqueous solution 100mml, add the sodium salt injection of 0.1mg aminodextran sulfate (sodium heparin, Changzhou Qianhong Biochemical Pharmaceutical Co., Ltd., molecular weight is 12 000-19 000 Daltons, in structural formula 1, n=20-32), deploy endoscope vision clearing agent 2 (mucopolysaccharide lipid content is about 0.1%, sodium chloride content is about 0.9%).
Embodiment 3
[0050] Add 0.1 mg of sodium hyaluronate injection (Alzhi, Japan Biochemical Industry Co., Ltd., molecular weight 5,000-10,000 Daltons, in structural formula 1, n=13-26) to 100 mml of medical sodium chloride aqueous solution, and prepare endoscopic Lens clearer 3 for mirror (about 0.1% mucopolyglycolipid content, about 0.9% sodium chloride content).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com