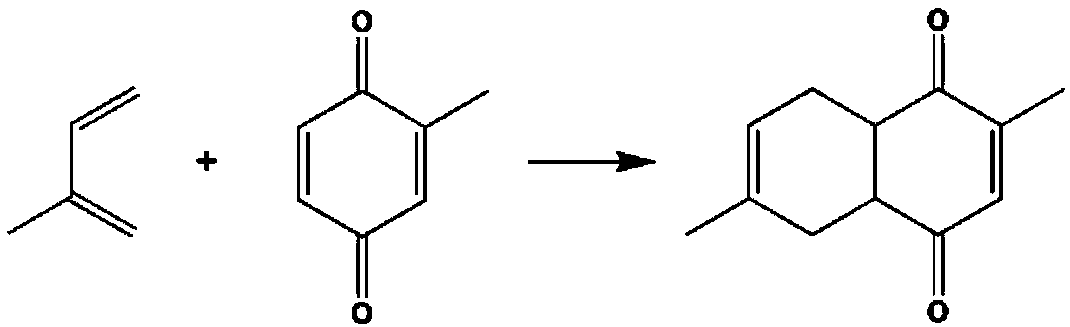
Method for preparing 2,6-dimethylnaphthalene from isoprene and methyl p-benzoquinone
A technology of isoprene and dimethylnaphthalene, which is applied in the field of preparing 2,6-dimethylnaphthalene, can solve the problems of complex raw material components of coal tar, low content of target products, harsh reaction system, etc., and achieve product yield High efficiency, short reaction route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
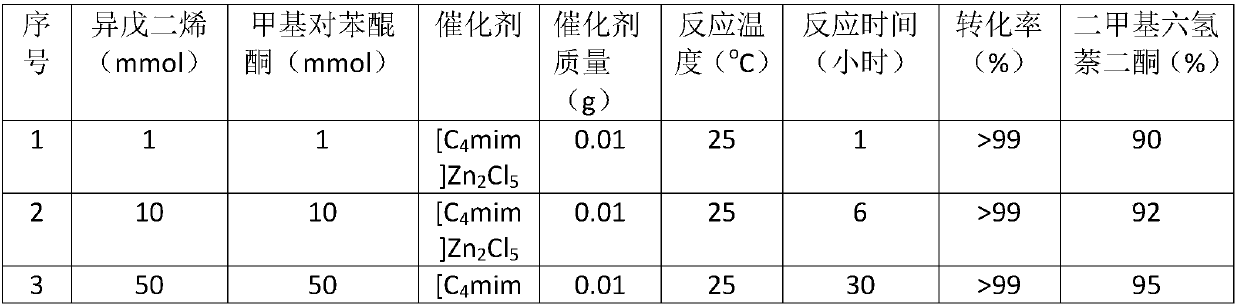
[0029] [C 4 mim]Zn 2 Cl 5 The preparation adopts the following method, respectively 1.72g ionic liquid [C 4 mim]Cl (10mmol) and 2.72g (20mmol)) of zinc chloride were added to a round-bottomed flask, stirred at 150°C for 2 hours, and cooled to room temperature after the reaction to obtain the final zinc-containing ionic liquid [C 4 mim]Zn 2 Cl 5 catalyst.
[0030] Other Lewis acid catalysts can directly use commercial catalysts.
Embodiment 2
[0032] Add a certain amount of isoprene, methyl-p-benzoquinone and Lewis acid catalyst into the hydrothermal reaction kettle, and react at different temperatures. After a certain period of time, take a sample and add about 1mL tetrahydrofuran to dilute, mix well, and centrifuge to separate the product. Qualitative analysis by GC-MS, quantitative analysis by GC external standard method. The reaction results are shown in Table 1.
[0033] Table 1. The reaction results of isoprene and methyl-p-benzoquinone under common heating conditions
[0034]
[0035]
[0036] The above results show that in the temperature range of 20°C-80°C, the ratio of isoprene / methyl-p-benzoquinone / catalyst is between 10-100, and the reaction is 20-90 hours, and dimethylhexahydronaphthalene di Ketone, the yield is between 60%-95%, it is better to use [C 4 mim]Zn 2 Cl 5 React at room temperature for 20-30 hours or heat to 40-50°C for 60-90 hours.
Embodiment 3
[0038]Add a certain amount of isoprene, methyl-p-benzoquinone and Lewis catalyst into the reaction tube, and react under microwave conditions of different powers. After a certain period of time, take a sample and add about 1ml tetrahydrofuran to dilute, mix well, and centrifuge to separate the product. Qualitative analysis by GC-MS, quantitative analysis by GC external standard method. The reaction results are shown in Table 2.
[0039] Table 2. Reaction results of isoprene and methyl-p-benzoquinone under microwave heating conditions
[0040]
[0041]
[0042] The above results show that between 200W-1000W of microwave power and 10-80 minutes of reaction, dimethyl hexahydronaphthalenedione can be obtained, and the yield is still between 60%-97%, preferably at 600-800W for 40 minutes. -60 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com