HPMA-polymer-modified-gold-nanorod medicine-carrying system and production method and application thereof
A gold nanorod, drug-carrying system technology, applied in pharmaceutical formulations, urinary system diseases, medical preparations with inactive ingredients, etc. The effect of increasing lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
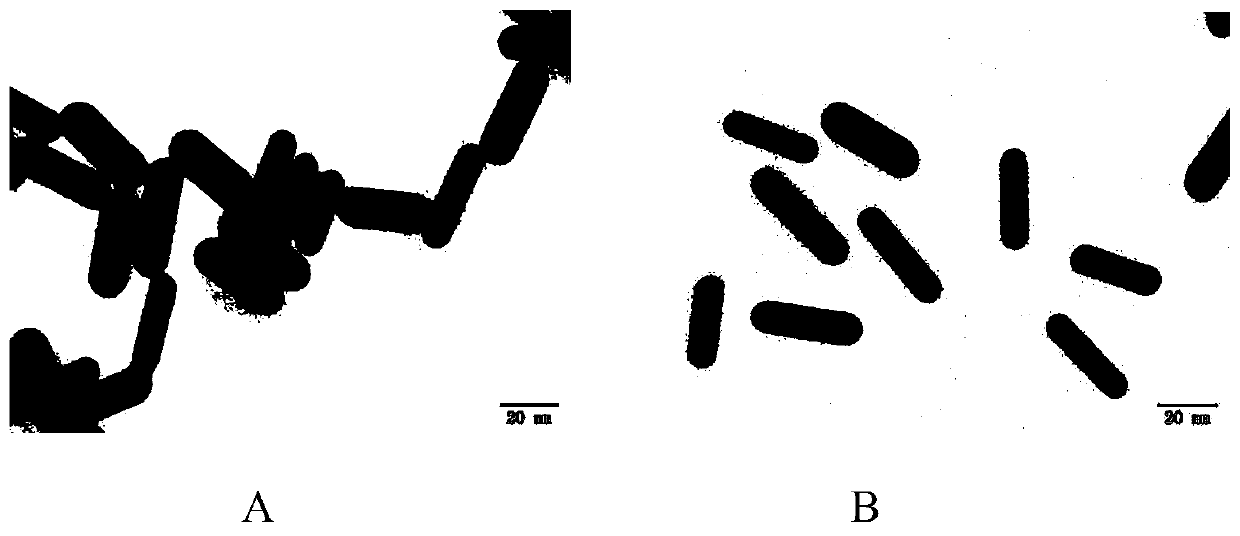
[0037] Preparation of CTAB-GNRs:
[0038](1) Preparation of seed solution: Add 5 mL, 0.0005 mol / L chloroauric acid solution to 5 mL, 0.2 mol / L cetyltrimethylammonium bromide (CTAB) solution in a water bath at 26 °C, and stir evenly Finally, 600 μL, 0.01mol / L ice-cold sodium borohydride solution was quickly added, the reaction solution changed from yellow to brown rapidly, stirred vigorously for 2 minutes, and stood at 26°C in the dark for 20 to 30 minutes.
[0039] (2) Preparation of growth solution: Add 200 μL, 0.004 mol / L silver nitrate (AgNO3) solution, 5 mL, 0.001 mol / L chloroauric acid solution to 5 mL, 0.2 mol / L CTAB solution in a water bath at 26 ° C, and stir Evenly, add 70 μL, 0.0788mol / L AA solution under slow stirring, the reaction solution changes from dark yellow to colorless quickly, quickly add 80 μL of the above seed solution, stir vigorously for 2 minutes, and stand overnight at 26°C in the dark to obtain CTAB-GNRs .
Embodiment 2
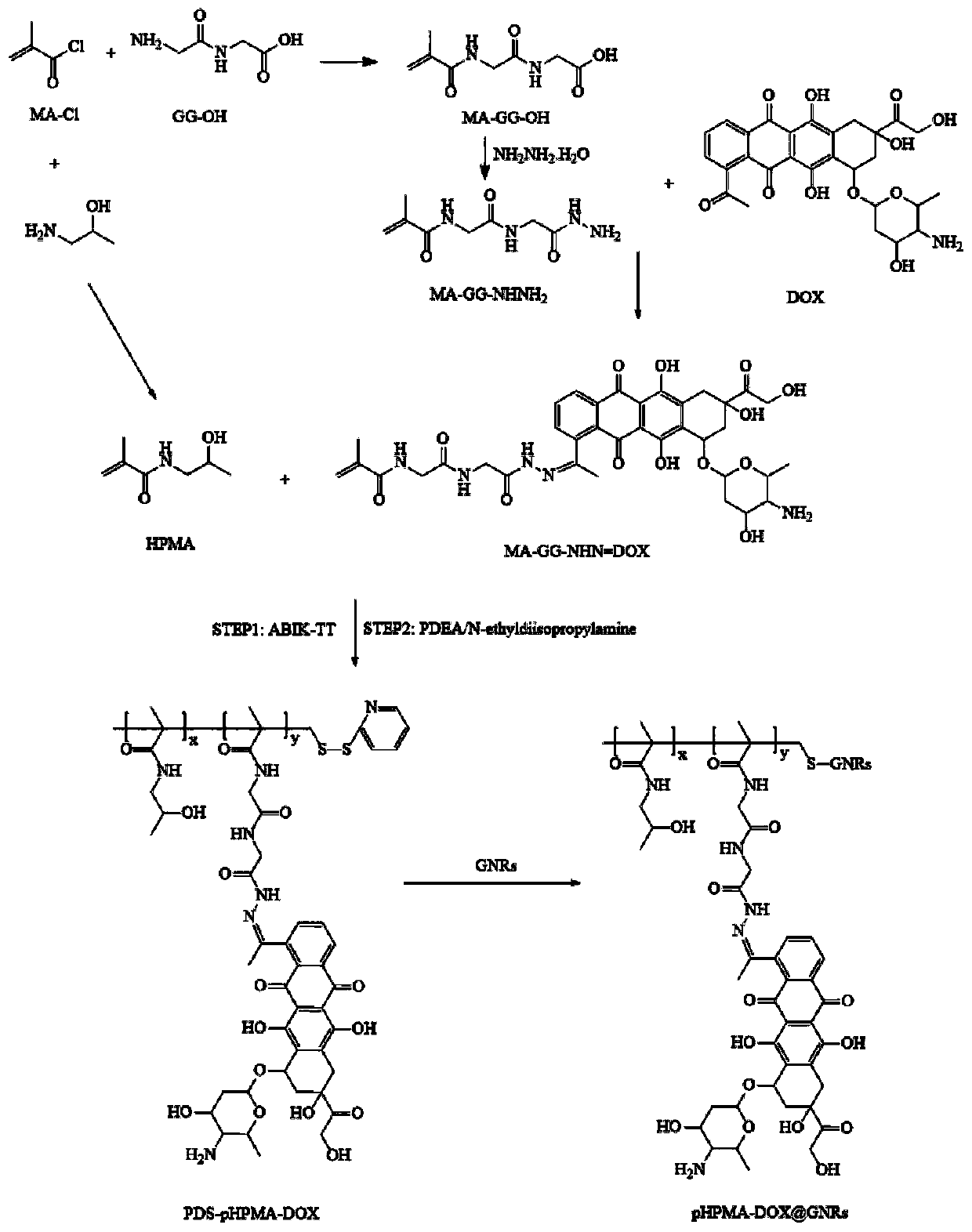
[0041] To prepare PDS-pHPMA-DOX, the synthetic route is as follows figure 1 As shown, the specific operation steps are as follows:
[0042] (1) Preparation of MA-GG-OH: Mix 8.71ml (0.091mol) of MA-Cl with 30ml of dichloromethane, dissolve 9.79g (0.075mmol) of GG-OH in 20ml of 4M sodium hydroxide solution, and store at -15°C Slowly drop the MA-Cl solution in dichloromethane into the GG-OH-containing sodium hydroxide solution while stirring, and at the same time drop into the 1M sodium hydroxide solution to adjust the pH to 9-10, and stir at room temperature for 1.5 h. After the dichloromethane layer was separated, it was washed with 20 ml of water, the aqueous layers were combined, and the pH was adjusted to 1-2 with 6M aqueous hydrochloric acid. The precipitate was recrystallized with 50% ethanol aqueous solution to obtain 6.44 g of white crystal MA-GG-OH, with a yield of 71.46%, M.p.136-138°C; MS: C 8 h 12 N 2 o 4 , 201.1 (M + ).
[0043] (2) Preparation of MA-GG-NHNH ...
Embodiment 3
[0048] To prepare pHPMA-DOX@GNRs, the synthetic route is as follows figure 1 As shown, the specific operation steps are as follows:
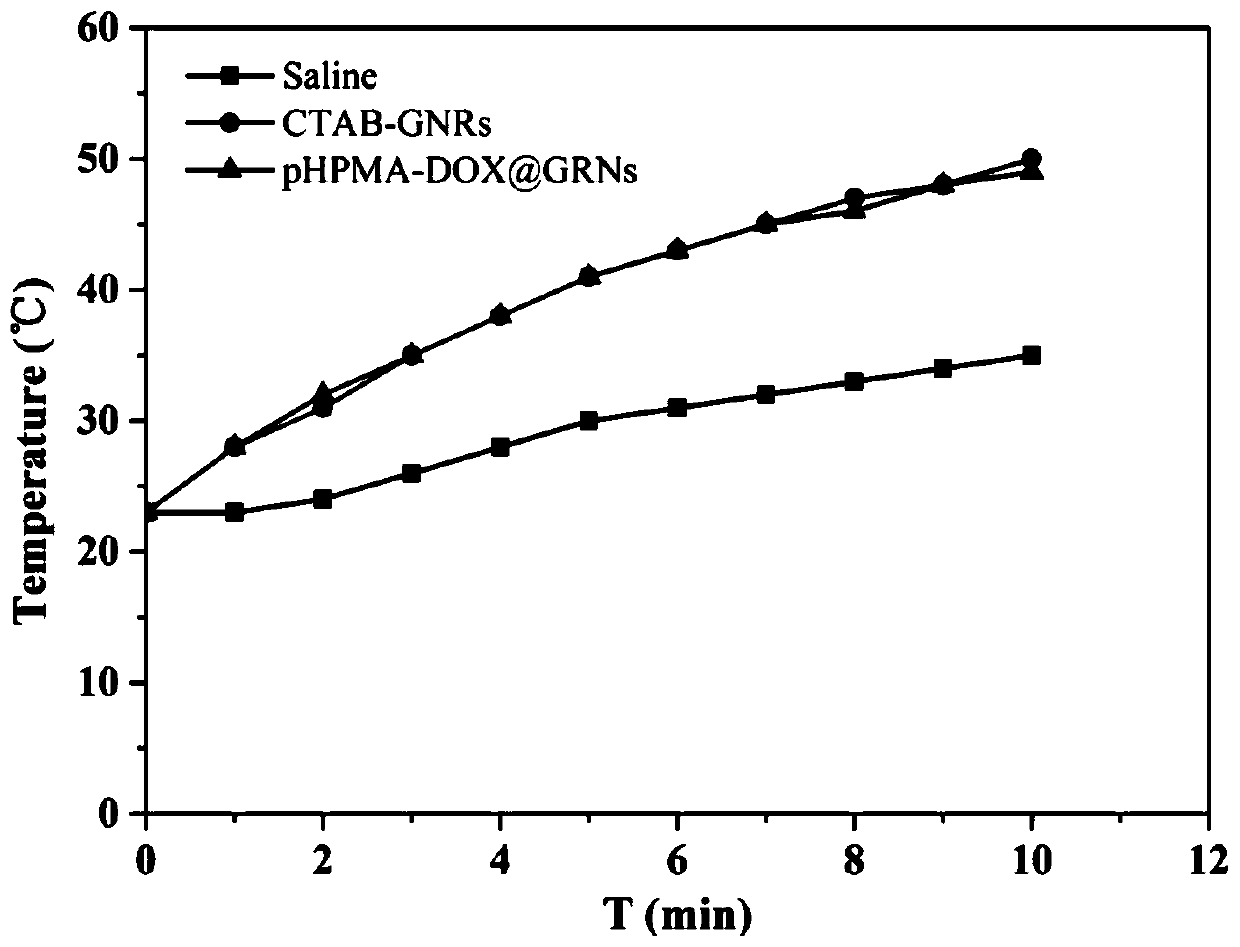
[0049] Take 1 mL (0.60 nM) of CTAB-GNRs prepared in Example 1 and resuspend in physiological saline, slowly add 1 mL (0.90 nM) of PDS-pHPMA-DOX in Example 2 dropwise under stirring conditions, and react in the dark for 12 hours to obtain 2 mL (0.3nM) product pHPMA-DOX@GNRs.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Horizontal length | aaaaa | aaaaa |
| Longitudinal length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com