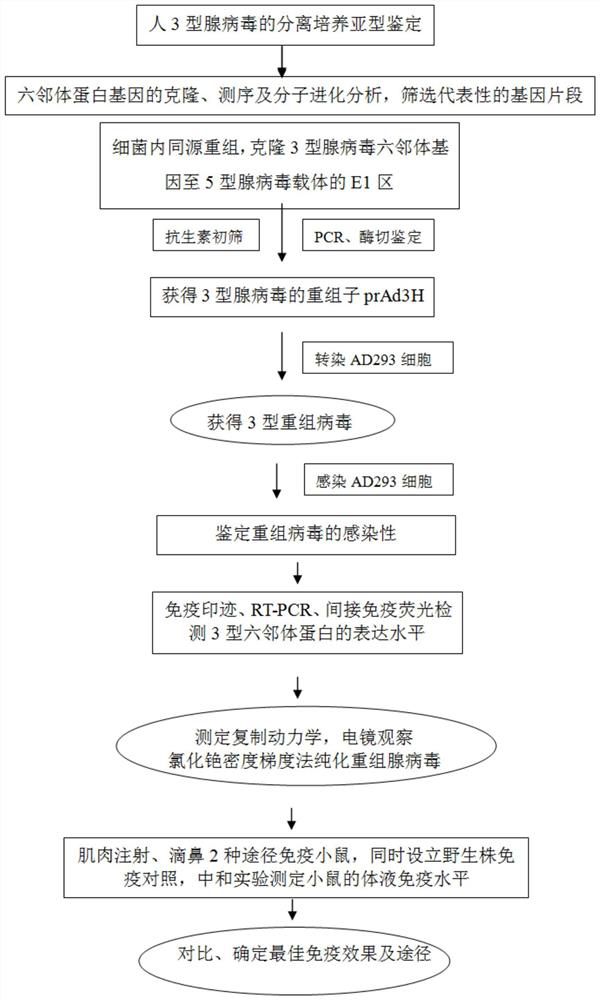
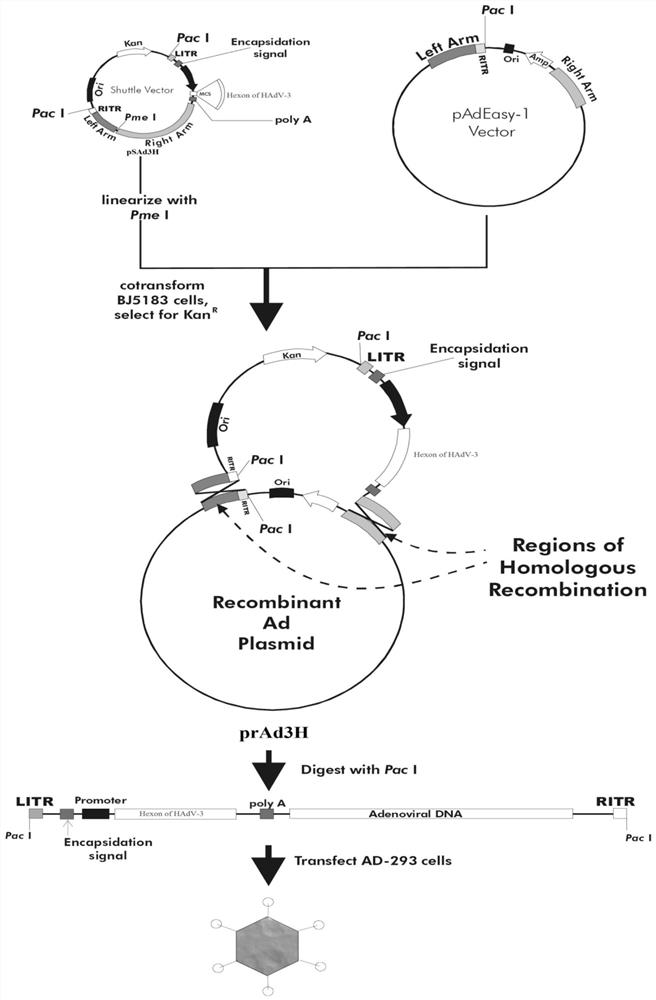
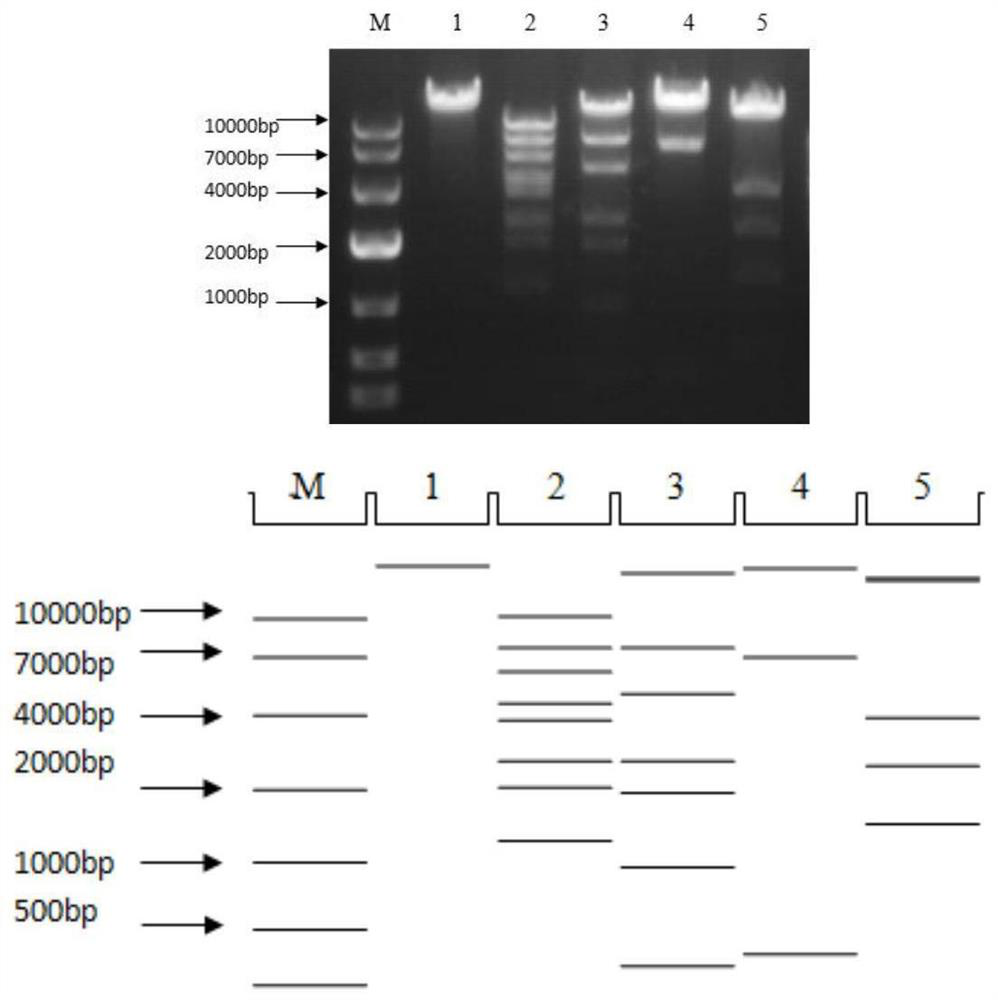
A kind of human type 3 adenovirus replication defective recombinant virus, construction method and application
A technology for replication-deficient recombinant viruses, applied in the field of construction of human type 3 adenovirus replication-defective recombinant viruses, can solve the problems of only symptomatic treatment and non-specific drugs, and achieve good application prospects and simple preparation methods , to ensure the effect of immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction of type 3 replication-deficient adenovirus and expression of target protein
[0039] 1. The construction process of the plasmid pSAd3H containing the hexon gene of human adenovirus type 3
[0040] The hexon gene of human type 3 adenovirus involved in the present invention is a complete human type 3 adenovirus hexon antigen gene, the nucleotide sequence is shown in SEQ ID NO.3, which comes from the wild type human adenovirus type 3 Strain GZ01 (GenBank: DQ099432). A pair of primers were designed according to the nucleotide sequence of the hexon protein of human type 3 adenovirus (HAdV-3GZ01 strain) and the restriction site in the multiple cloning site of the shuttle plasmid pShuttle-CMV-GFP:
[0041] The upstream outer primer sequence is the sequence shown in SEQ ID No.1 (Ad3-GZ01-EcoRV-HexF: TGAGATATCGCCACCATGGCCACCCCATC);
[0042] The downstream outer primer sequence is the sequence shown in SEQ ID No. 2 (Ad3-GZ01-HexR-XhoI: AGACTCGAGTTATGTGGTG...
Embodiment 2
[0101] Example 2 Immunogenicity of type 3 replication-deficient adenovirus
[0102] A large amount of replication-deficient adenovirus type 3 was amplified, and the virus was purified by the cesium chloride density gradient method. The operation was as follows:
[0103] (1) Harvesting cells: Repeated freezing and thawing 3 times of AD293 cells with 90% CPE in culture, centrifuging at 1000g for 10min, collecting the supernatant, and freezing at -80°C; (2) discontinuous density gradient centrifugation of the virus (all operations below are Take a 30mL centrifuge tube as an example): a. Pre-cool the rotor of the ultracentrifuge to 4°C. b. Slowly add 8mL 1.4g / mL cesium chloride to the centrifuge tube of the ultra-high speed centrifuge, and then add 6mL 1.2g / mL cesium chloride very gently; c. Add at the top of the discontinuous gradient cesium chloride solution 20mL virus collection solution, if the volume of virus solution is less than 20mL, use pH 7.9 10mmol / L Tris-HCl to balanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com