Application of additive in negative electrode electrolyte of alkaline zinc-nickel flow battery
A negative electrode electrolyte and flow battery technology, applied in fuel cells, regenerative fuel cells, circuits, etc., can solve problems such as hydrogen evolution side reactions, achieve improved battery performance, poor short-term high-efficiency cycle stability, and improve Coulombic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add the lead nitrate that final concentration is 0.03mol / L with the negative electrode electrolyte of comparative example 1, battery 40mA / cm 2 See Table 1 for running performance indicators.
[0020] As shown in Table 1, after the introduction of divalent lead ions into the negative electrode electrolyte of the battery, the coulombic efficiency of the battery increases, and the coulombic efficiency of the battery decreases after the battery runs to 400 cycles.
[0021] The additive has higher hydrogen evolution overpotential and deposition potential higher than zinc ion deposition potential. During the charging process of the battery, a small amount of side reaction of hydrogen evolution will occur at the negative electrode, which will affect the performance of the battery. After adding this additive, at the initial stage of charging, the divalent lead ions in the solution will preferentially obtain electrons and become lead simple substance and deposit on the negative ...
Embodiment 2
[0025] The difference from Comparative Example 1 is that: tin nitrate with a final concentration of 0.03mol / L is added to the negative electrode electrolyte, and the battery is 40mA / cm 2 See Table 2 for running performance indicators.
[0026] As shown in Table 2, after introducing divalent tin ions into the negative electrode electrolyte of the battery, the coulombic efficiency of the battery is significantly improved.
[0027] The additive has stable properties in alkaline solution, high hydrogen evolution overpotential and deposition potential higher than zinc ion deposition potential. During the charging process of the battery, a small amount of side reaction of hydrogen evolution will occur at the negative electrode, which will affect the performance of the battery. After adding this additive, at the initial stage of charging, the divalent tin ions in the solution will preferentially obtain electrons and become simple tin to deposit on the negative electrode. It is very ...
Embodiment 3
[0031] The difference from Comparative Example 1 is that: lead nitrate with a final concentration of 0.015mol / L and tin nitrate with a final concentration of 0.015mol / L are added to the negative electrode electrolyte, and the battery is 40mA / cm 2 The operating performance indicators are shown in Table 3.
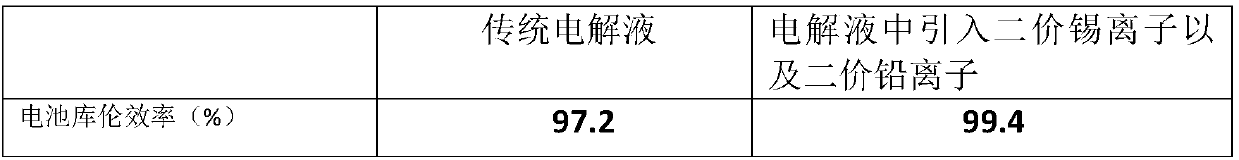
[0032] As shown in Table 3, after introducing divalent lead ions and divalent tin ions into the battery negative electrolyte, the coulombic efficiency of the battery is higher than when no other ions are introduced, lead ions and tin ions are introduced alone.
[0033] The additive has stable properties in alkaline solution, high hydrogen evolution overpotential and deposition potential higher than zinc ion deposition potential. During the charging process of the battery, a small amount of side reaction of hydrogen evolution will occur at the negative electrode, which will affect the performance of the battery. After adding this additive, at the initial stage of charging, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com