Synthesis method of gatifloxacin-chalcone conjugate slow release agent
A technology of gatifloxacin and its synthesis method, which is applied in the directions of pharmaceutical formulation, drug combination, drug delivery, etc., can solve problems such as failure and short retention time, and achieve the effect of promoting inhibition, improving water solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A synthetic method of gatifloxacin and chalcone conjugate slow-release agent, comprising the following steps:
[0028] S1. Preparation of gatifloxacin and chalcone conjugates;
[0029] S2. Ginkgo biloba polyprenol and chitosan were mixed and dissolved in 0.2mol / L acetic acid solution at a molar ratio of 2:1, using Cu as a catalyst, and reacted at 180°C for 2 hours to obtain composite chitosan;
[0030] S3. Dissolve the gatifloxacin and chalcone conjugate obtained in step S1 and the complex chitosan obtained in step S2 in a hydrochloric acid solution with a mass fraction of 0.5% according to a mass ratio of 1:30, and Ultrasound 20min;
[0031] S4. Adjust the mixed solution obtained in step S3 to be neutral, and spray dry it.
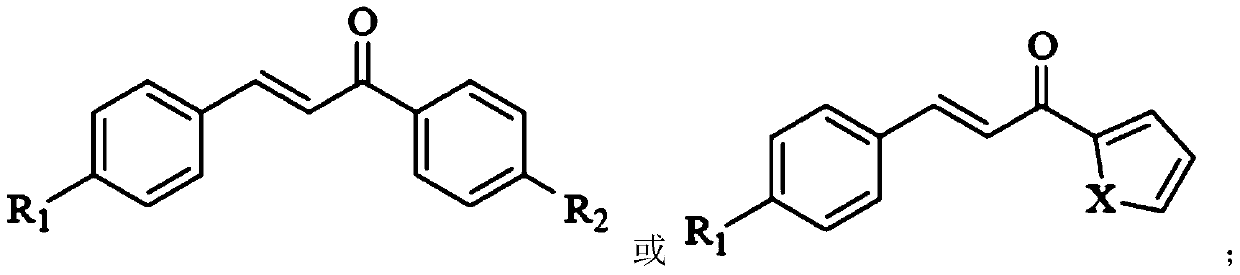
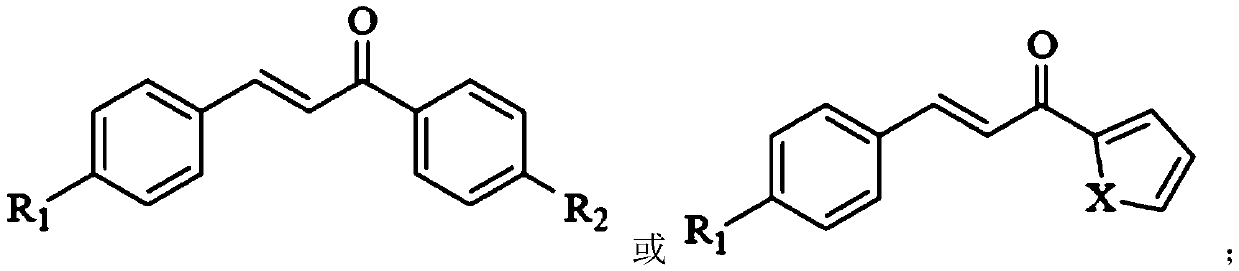
[0032] The structure of the chalcone is:
[0033]
[0034] R1 is Br and R2 is H.
Embodiment 2
[0036] A synthetic method of gatifloxacin and chalcone conjugate slow-release agent, comprising the following steps:
[0037] S1. Preparation of gatifloxacin and chalcone conjugates;
[0038] S2. Ginkgo biloba polyprenol and chitosan were mixed and dissolved in 0.5mol / L acetic acid solution at a molar ratio of 5:1, using Cu as a catalyst, and reacted at 160°C for 1 hour to obtain composite chitosan;
[0039] S3. Dissolve the gatifloxacin and chalcone conjugate obtained in step S1 and the complex chitosan obtained in step S2 in a hydrochloric acid solution with a mass fraction of 0.1% according to a mass ratio of 1:50, and Ultrasound for 30min;
[0040] S4. Adjust the mixed solution obtained in step S3 to be neutral, and spray dry it.
[0041] The structure of the chalcone is:
[0042]
[0043] R1 is Br, and X is S.
Embodiment 3
[0045] A synthetic method of gatifloxacin and chalcone conjugate slow-release agent, comprising the following steps:
[0046] S1. Preparation of gatifloxacin and chalcone conjugates;
[0047] S2. Ginkgo polyprenol and chitosan were mixed and dissolved in 0.3mol / L acetic acid solution at a molar ratio of 4:1, and Cu was used as a catalyst to react at 170°C for 1 hour to obtain composite chitosan;
[0048] S3. Dissolve the gatifloxacin and chalcone conjugate obtained in step S1 and the complex chitosan obtained in step S2 in a hydrochloric acid solution with a mass fraction of 0.2% according to a mass ratio of 1:45, and Ultrasound for 25 minutes;
[0049] S4. Adjust the mixed solution obtained in step S3 to be neutral, and spray dry it.
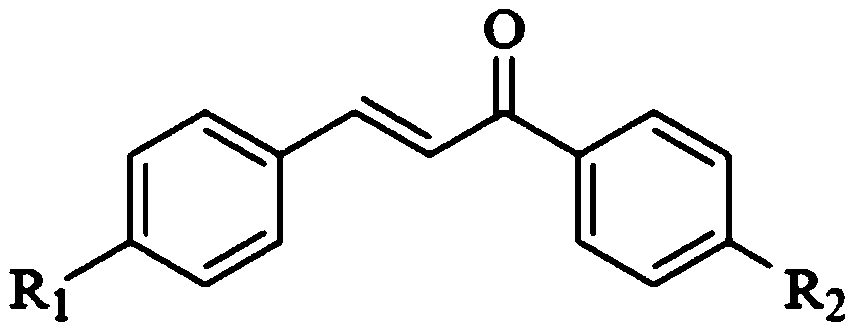
[0050] The structure of the chalcone is:
[0051]
[0052] R1 is Br, and X is N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com