Novel application of hyaluronic acid fragment and stable making method
A technology of hyaluronic acid and hyaluronidase, applied in the field of biomedicine, can solve the problems such as the stable manufacturing method of bioactive hyaluronic acid fragment B-HA, the limited research on the new clinical application of the manufacturing principle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Objective: To study the stable manufacturing method and principle of using different hyaluronidases to fully or slightly excessively enzymatically hydrolyze high or medium molecular hyaluronic acid raw materials to produce low molecular hyaluronic acid fragments.
[0053] method:
[0054]1. The methods for measuring the molecular weight of hyaluronic acid and hyaluronic acid fragments used in the present invention include electrophoretic measurement methods and 18-angle laser measurement methods. Manufacturers of high or medium molecular weight hyaluronic acid raw materials and the present invention adopt viscometer and 18-angle laser to measure.
[0055] 2. Hyaluronic acid raw materials: hyaluronic acid raw materials with an average molecular weight of 300kDa (medium molecular weight) and 1600kDa (high molecular weight) (Huaxi Freda Biotechnology Co., Ltd.).
[0056] 3. Hyaluronidase: recombinant human hyaluronidase PH20 (Shaoxing Huihui Technology Co., Ltd.), bovine ...
Embodiment 2
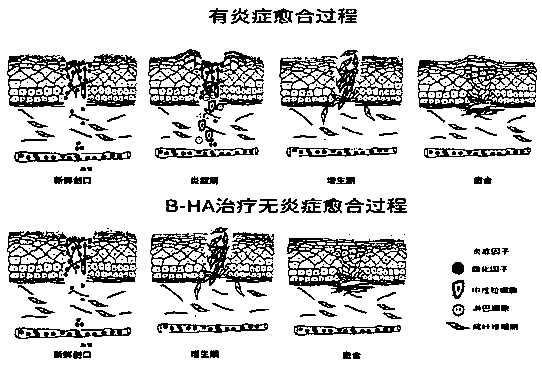
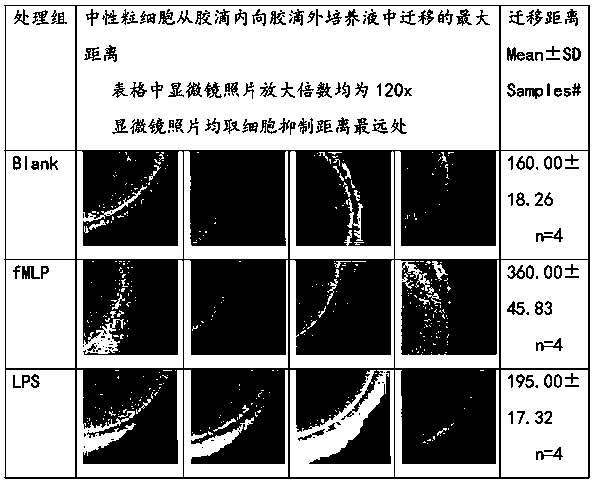
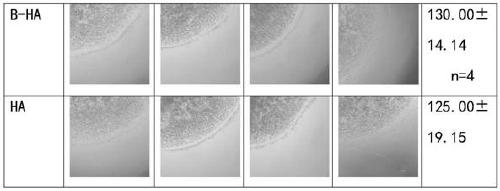
[0103] Objective: To study the effect of using recombinant human hyaluronidase PH20 in sufficient or slight excess to fully enzymatically hydrolyze high-molecular-weight and medium-molecular-weight hyaluronic acid raw materials to stably produce hyaluronic acid fragments (hereinafter referred to as B-HA) with an average molecular weight of 35±8KDa. Effects of extracted human leukocytes (neutrophils and mononuclear cells) on removal, phagocytosis, apoptosis, TNFɑ secretion, IL-6 secretion and beta-defensin secretion.
[0104] result:
[0105] 1. The effect of B-HA on the secretion of inflammatory factor TNFɑ from neutrophils
[0106] Table 1. B-HA did not significantly promote or inhibit the secretion of TNFɑ from human neutrophils in the presence or absence of erythrocytes (p>0.05; p>0.05). Similar results were obtained using freshly extracted human neutrophils from different healthy volunteers.
[0107] Table 1. Effect of B-HA on secretion of TNF by human neutrophils in the...
Embodiment 3
[0176] Objective: To use commercial hyaluronidase PH20 injection and macromolecular hyaluronic acid injection to produce B-HA or HA35 with an average molecular weight of 35.4kDa in a short period of time to treat the high-sensitivity inflammatory response of local large-area redness, swelling and hard pain caused by mosquito bites .
[0177] method:
[0178] Twelve cases of patients with hypersensitivity reaction to mosquito (mosquito or bumblebee) bites (with rapid localized large-area redness, swelling and pain in the bite area). Use the following injections to inject into the affected area outside the instructions, and then observe the changes in the large area of red, swollen and hard pain in the bite area after treatment.
[0179] Before injection, the recombinant human hyaluronidase PH20 injection (Hylenex, 150unit / mL injectionsolution, Hylozyme Inc) and Durolane 20mg / ml, 3ml / Vial (Genvrier) with an average molecular weight of 1600kDa were prepared according to the ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com