Novel application of intestinal alkaline phosphatase and product activity quality control method of preparation of intestinal alkaline phosphatase
A phosphatase, a new application technology, applied in the direction of medical preparations containing active ingredients, non-central analgesics, biochemical equipment and methods, etc., can solve the research on the biological activity, physiological function and clinical application of alkaline phosphatase Limited and other problems, to achieve the effect of inhibiting inflammation and inhibiting removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
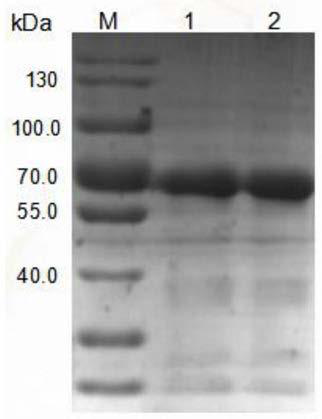
[0057] Purpose: Preparation of recombinant human intestinal alkaline phosphatase
[0058] Methods: A chimeric recombinant human intestinal alkaline phosphatase (recIAP) cDNA (references 24, 25) was used to construct the expression vector pMH3-recIAP (reference 26), and then the constructed pMH3-recIAP was transformed into CHO-S Cells (CVCL_7183, Life Technologies). Use DMEM / F12 culture medium containing 10% fetal bovine serum (FBS) to obtain stable and high-expression cell clones through G418 pressurized screening as seed cell lines for harvesting proteins, expand culture in animal cell bioreactors, and obtain harvest liquid for purification For treatment, the harvest liquid was centrifugally concentrated and added to an anion column (Q XK50, GE Healthcare), and the eluate containing high-concentration recIAP was collected and depyrogenated through a hollow fiber column (Borgeron Biotechnology Co., Ltd.) for later use. The purity of the solution containing high concentration ...
Embodiment 2
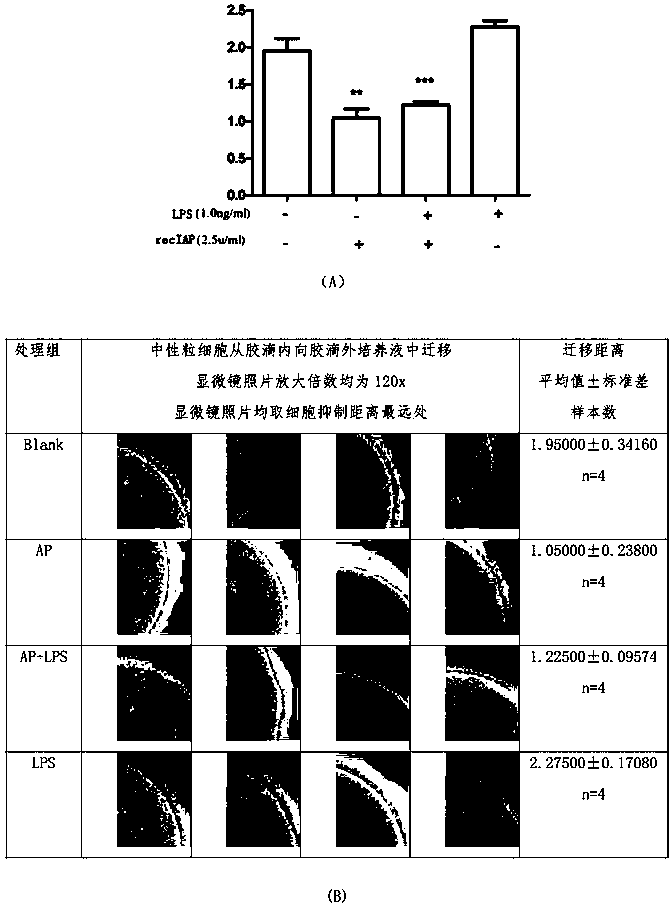
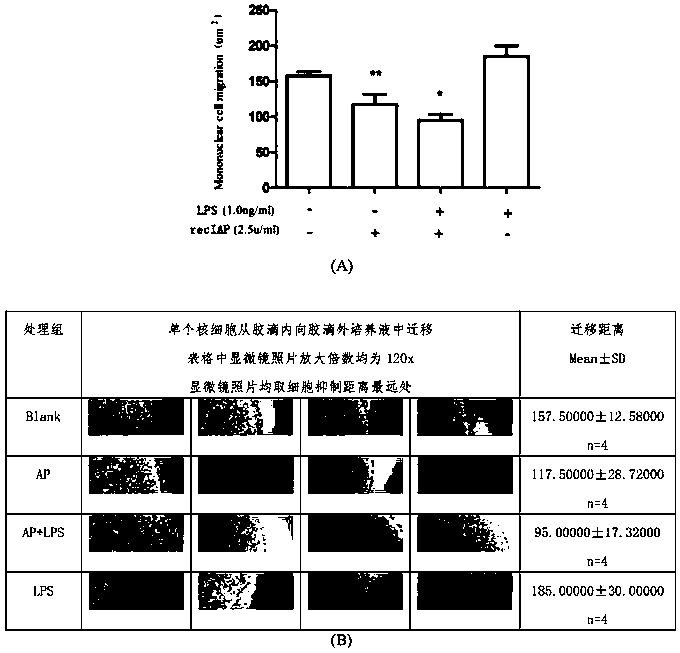
[0065] Objective: Intestinal alkaline phosphatase inhibits human neutrophil emigration
[0066] method:
[0067] 1. Isolation of Neutrophils and Mononuclear Cells from Human Venous Blood
[0068] A total of 6 healthy volunteers, aged 26±5 years old, blood collection was approved by the Medical Ethics Committee of Changchun Jiahe Surgical Hospital and I agreed. The present invention adopts the sugar density gradient centrifugation method and the human venous blood leukocyte separation kit (endotoxin<0.1EU) (Tianjin Haoyang Huake Biotechnology Co., Ltd.) to separate venous blood. Collect human venous blood at room temperature and centrifuge at 1800rpm for 25 minutes, absorb the mononuclear cell layer (lymphocytes and a small number of monocytes) and the multinuclear cell layer (mainly neutrophils) to mix, fully lyse the red blood cells, wash twice, and use 3ml Resuspended in 1640 medium containing 10% FBS for use. The cell morphology was observed by staining with leukocyte di...
Embodiment 3
[0074] Objective: Study on Phagocytosis of Fluorescent Particles by Human Neutrophils
[0075] Methods: Human neutrophils were adjusted to 2×106 cells / ml in RPMI-1640 medium containing 10% FBS. Add 200ul cells per well, inoculate in a 24-well plate, add 5U / ml recIAP and 1ng / ml LPS separately or simultaneously to stimulate neutrophils. Add 3.5ul of carboxylate-modified polystyrene (L3030 Sigma-Aldrich) to each well of latex beads with a diameter of 2um, adjust the density of fluorescent particles to 2×107 / ml, and establish the best phagocytosis model of neutrophils and fluorescent particles After phagocytosis culture at 37°C for 1 h, flow cytometer (FACSCalibur US PE Company) 488nm wavelength laser collects red fluorescence with a wavelength of 575nm to obtain the phagocytosis rate of neutrophils, so as to study the phagocytosis ability of neutrophils. After the experiment is completed, freshly extract another human venous blood leukocytes to repeat the experiment to ensure th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com