Novel drug-eluting balloon catheter and preparation method thereof
A technology for balloon catheters and drugs, applied in catheters, pharmaceutical formulations, drug delivery, etc., can solve the problems of difficult reprinting of drugs, strong adhesion between hydrophobic coating and balloon surface, etc., to facilitate industrial operation and improve Bioavailability, effect with small batch-to-batch variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
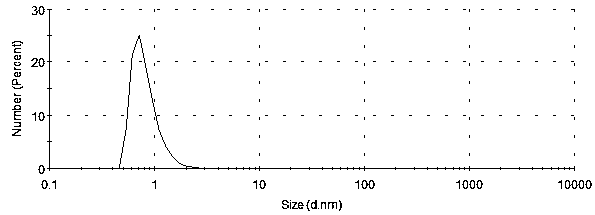
[0042]Weigh 10mg of rapamycin, disperse it in 20ml of water, add this solution dropwise to 50ml of PVA solution with a mass fraction of 3%, and use ultrasonic emulsification at 4°C for 40 minutes, then stir the emulsion overnight at a speed of 1000rpm. The overnight emulsion was centrifuged at 3500rpm, and the supernatant was taken to obtain a suspension of rapamycin microspheres. The suspension was centrifuged at 12000rpm for 30 minutes, then the supernatant was discarded, and 5 % Span 85 emulsifier solution 2ml, repeat this centrifugation step 2 times and adopt nano particle size analyzer to test the size and distribution of solid microsphere particles, the particle size range is 0.3nm-3nm, the result is as follows figure 1 .
Embodiment 2
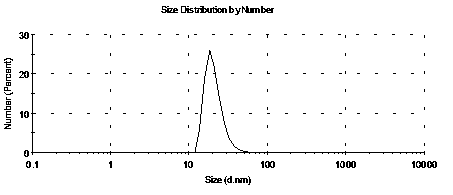
[0044] Weigh 10mg of rapamycin, dissolve it in 10ml of acetone, add this solution dropwise to 50ml of PVA solution with a mass fraction of 1%, use ultrasonic emulsification at 4°C for 40 minutes, then stir the emulsion overnight at a speed of 1000rpm, The overnight emulsion was centrifuged at 2000 rpm, and the supernatant was taken to obtain a suspension of rapamycin microspheres (also referred to as a suspension, the same below). Centrifuge the suspension at 10,000rpm for 30 minutes, then pour off the supernatant, add 2ml of 1% cetyl alcohol emulsifier solution, repeat this centrifugation step 3 times, and use a nanometer (Zetasizer Nano ZS90) to test the solid microspheres The size and distribution of particles, the particle size range is 10nm-90nm, the results are as follows figure 2 .
Embodiment 3
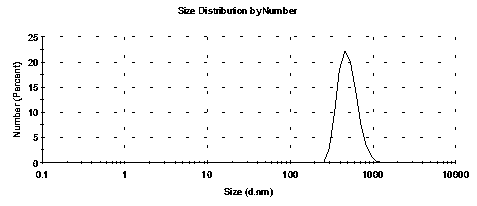
[0046] Weigh 10 mg of rapamycin, disperse it in 10 ml of water, add this solution dropwise to 50 ml of polysorbate 20 solution with a mass fraction of 3%, and use ultrasonic emulsification at 4°C for 40 minutes, then stir the emulsion overnight, at a speed of centrifuge the overnight emulsion at 3500rpm, and take the supernatant to obtain a suspension of rapamycin microspheres, centrifuge the suspension at 12000rpm for 30 minutes, then pour off the supernatant , add 2ml of 5% Span 80 emulsifier solution, repeat this centrifugation step twice and use a nanometer to test the size and distribution of solid microsphere particles, the particle size range is 200nm-600nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com