Nitrogen-containing discotic ionic liquid crystal compound and preparation method thereof
A technology of ionic liquid crystals and compounds, applied in chemical instruments and methods, liquid crystal materials, organic chemistry, etc., can solve the problems of complex synthesis and tedious characterization of discotic ionic liquid crystals, and achieve good application value and prospects, and the effect of good luminescent properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
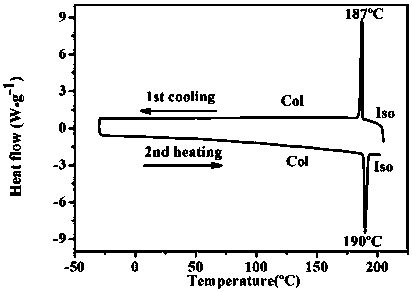
[0050] A specific embodiment of the present invention is: a nitrogen-containing discotic ionic liquid crystal compound, which has the structure of the following general formula I, wherein R 1 =C 6 h 13 , R 2 =C 6 h13 , X is an organic anion bistrifluoromethanesulfonimide ion.
[0051]
[0052] R in formula I 1 =C 6 h 13 , R 2 =C 6 h 13 , X is the preparation reaction formula of the nitrogen-containing discotic ionic liquid crystal compound of organic anion bistrifluoromethanesulfonimide ion as follows:
[0053]
[0054] In the reaction formula, THF is tetrahydrofuran; toluene is toluene; Tf 2 NLi is lithium bistrifluoromethanesulfonylimide.
[0055] The preparation of 3,4-dihexylphenylboronic acid is a prior art, see literature: Chun-Xia Liu, Hu Wang, Jun-Qi Du, Ke-Qing Zhao, Ping Hu, Bi-Qin Wang, Hirosato Monobe, BenoÎt Heinrich and Bertrand Donnio. Molecular design of benzothienobenzothiophene-cored columnar mesogens: facile synthesis, mesomorphism, and cha...
Embodiment 2
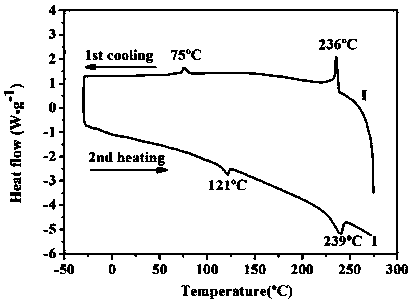
[0070] R in formula I 1 =C 6 h 13 , R 2 =C 6 h 13 , X is the preparation reaction formula of the nitrogen-containing discotic ionic liquid crystal compound of organic anion dodecylsulfonate ion as follows:
[0071]
[0072] Concrete preparation steps are as follows:
[0073] A. Suzuki coupling reaction
[0074] Add 1 molar part of 3,4-dibromopyridine, 2.5 molar parts of 3,4-dihexylphenylboronic acid, 10 molar parts of potassium carbonate, and 0.25 molar parts of tetrakis(triphenylphosphine)palladium to THF and h 2 In the mixed solution of O, under the protection of argon, the reaction was stirred at 70 ° C for 36 hours. After the reaction was completed, it was extracted with dichloromethane, the organic phase was dried with anhydrous magnesium sulfate, filtered, concentrated, and the concentrated residue was used for silica gel column layer Analysis, separation and purification (mobile phase: dichloromethane: ethyl acetate volume ratio = 10: 1) to obtain a viscous c...
Embodiment 3
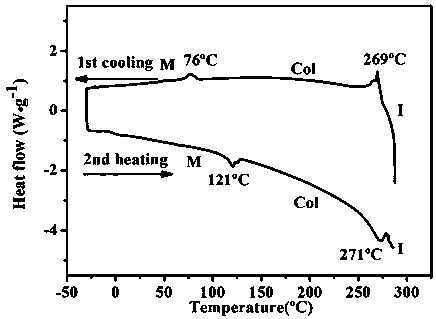
[0086] R in formula I 1 =C 6 h 13 , R 2 =C 6 h 13 , X is the preparation reaction formula of the nitrogen-containing discotic ionic liquid crystal compound of chiral organic anion L-camphorsulfonate ion as follows:
[0087]
[0088] Concrete preparation steps are as follows:
[0089] A. Suzuki coupling reaction
[0090] Add 1 molar part of 3,4-dibromopyridine, 2.5 molar parts of 3,4-dihexylphenylboronic acid, 10 molar parts of potassium carbonate, and 0.25 molar parts of tetrakis(triphenylphosphine)palladium to THF and h 2 In a mixed solution of O, under the protection of argon, the reaction was stirred at 70°C for 24 hours. After the reaction was completed, it was extracted with dichloromethane, the organic phase was dried with anhydrous magnesium sulfate, filtered, concentrated, and the concentrated residue was applied to a silica gel column layer Analysis, separation and purification (mobile phase: dichloromethane: ethyl acetate volume ratio = 10: 1) to obtain a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com