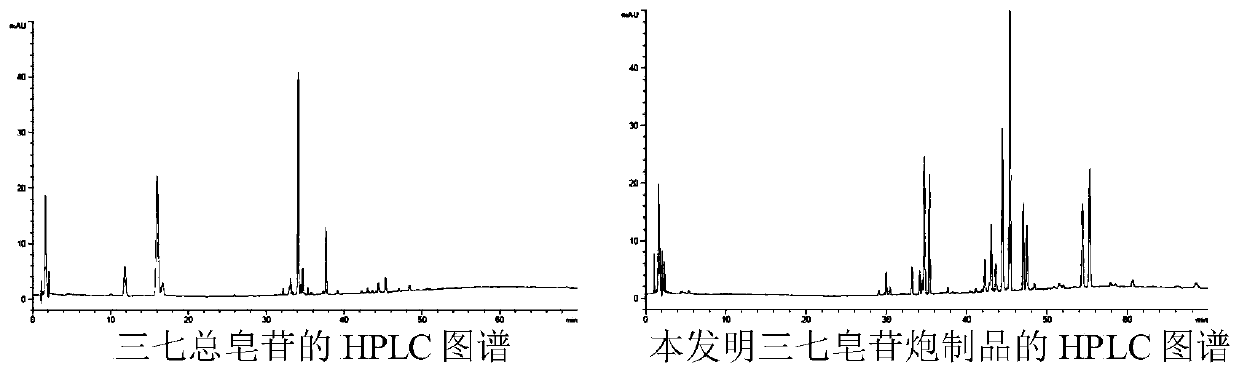
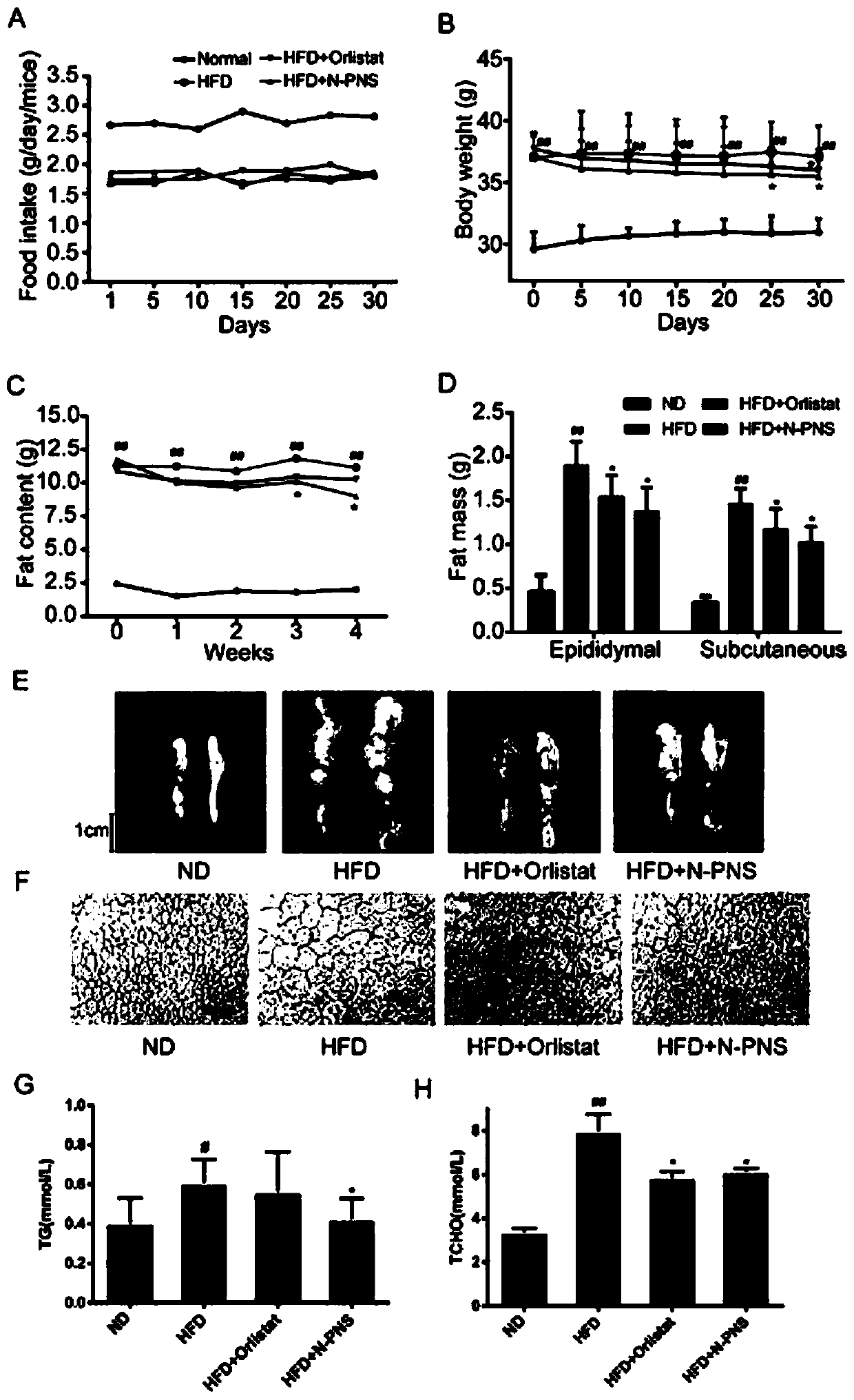
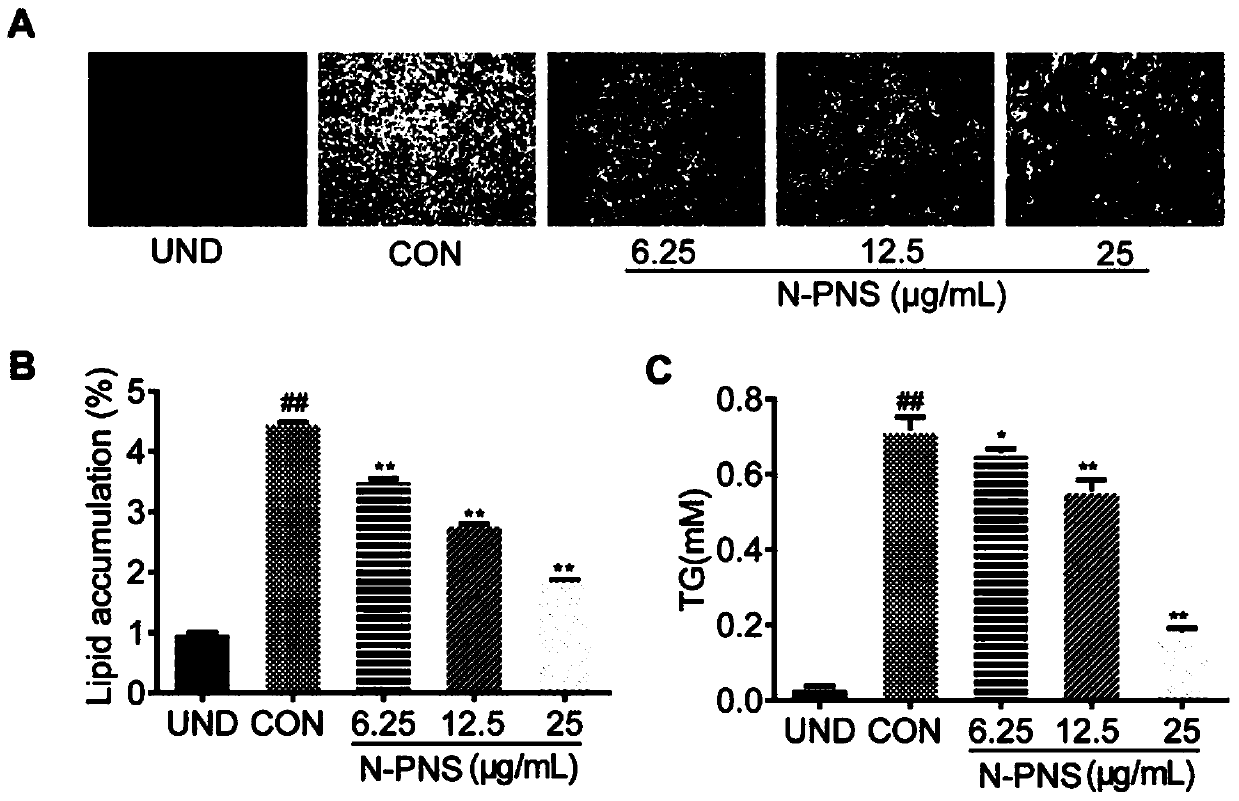
Notoginsenoside processed product as well as preparation method and application thereof
A technology of notoginseng saponin and saponin cannon, which is applied in the processed product of notoginseng saponin and its preparation, and its application in the preparation of medicines for treating obesity and hyperlipidemia, improving the application field of obesity and hyperlipidemia health care products or food, and reducing the content, lowering the body's blood fat, weight loss and blood fat lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Prepare notoginseng saponin processed product:
[0032] Take 10 grams of Panax notoginseng saponins, add 400ml of purified water and ultrasonically heat to assist in dissolution. Pour the fully dissolved sample into the hydration reaction kettle. Subsequently, the hydration reaction kettle was placed in a constant temperature drying oven, and the reaction was timed for 27 hours when the temperature of the drying oven rose to 120° C., and the reaction solution was poured out after the outer wall of the hydration reaction kettle cooled to normal temperature. The reaction solution was filtered and added to a chromatographic column equipped with D101 resin that had been equilibrated with purified water. First elute with water for 4-5 column volumes, then drain the column until no water drips from the column. Then add 10 times the column volume of more than 95% ethanol to elute. The 95% ethanol eluted part was collected, transferred to a rotary evaporator, and the tempera...
Embodiment 2
[0036] The processed product of notoginseng saponins of the present invention can be further made into medicines in different dosage forms, for example as follows:
[0037] 1. Tablet
[0038] Using wet granulation, weigh 10mg of processed product powder of notoginseng saponin, 50-100mg of lactose monohydrate and 25-50mg of microcrystalline cellulose, add 1.2mg of carboxymethylcellulose sodium, pass through a 60-mesh sieve, mix, repeat twice , add povidone 10-50mg to granulate, pass through a 20-mesh sieve, place in a blast drying oven at 55°C to dry, then add 1mg of sodium carboxymethylcellulose and 1-5mg of magnesium stearate, mix, and press with a tablet machine piece.
[0039] 2. Capsules
[0040] Weigh 10 mg of notoginseng saponin processed product powder, 50-100 mg of lactose monohydrate and 25-50 mg of microcrystalline cellulose, add 1.2 mg of sodium carboxymethyl cellulose, pass through a 60-mesh sieve, mix, repeat twice, add povidone 10-50 mg is granulated, passed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com