A kind of detection method of impurities in parecoxib sodium synthesis process
A parecoxib sodium and synthesis process technology, which is applied in the field of drug analysis and detection, can solve the problems of excessive sulfonylation derivative impurities without comprehensive detection, a large number of methods, and high analysis costs, and achieve wide coverage and separation The effect of good effect, high sensitivity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
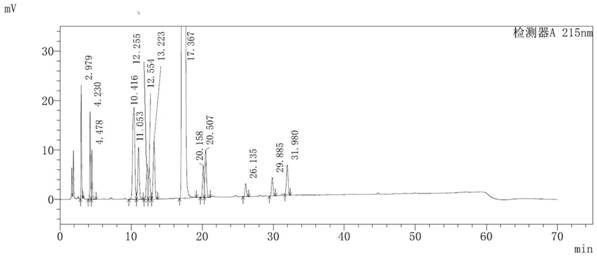
[0044] The method for detecting impurities in the parecoxib sodium synthesis process of the present embodiment adopts the following steps:
[0045] (1) Take an appropriate amount of the sample of parecoxib sodium to be tested, accurately weigh it, add acetonitrile-water (40:60, v / v) to dissolve and quantitatively dilute to prepare 0.5 mg of parecoxib sodium per 1 mL to be tested The solution of the sample, as the solution to be tested;
[0046] (2) Take 10 μL of the solution to be tested and inject it into a liquid chromatograph, record the chromatogram, and calculate the content of impurities in each process according to the standard curve method.
[0047] The chromatographic conditions for liquid phase detection are: chromatographic column: YMC-Pack ODS-AQ (4.6×250mm, 5μm); mobile phase A: 0.01mol / L disodium hydrogen phosphate solution (adjust pH to 3.0 with phosphoric acid); mobile phase B: acetonitrile-methanol (75:25, v / v); detection wavelength: 215 nm; column temperatur...
Embodiment 2
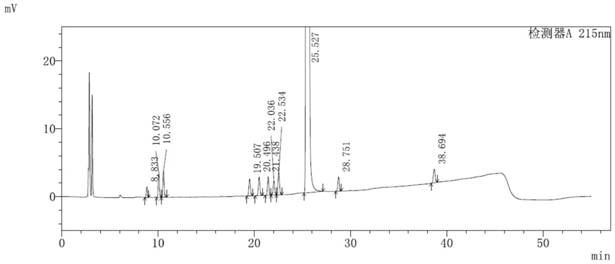
[0054] The method for detecting impurities in the parecoxib sodium synthesis process of the present embodiment adopts the following steps:
[0055] (1) Take an appropriate amount of the sample of parecoxib sodium to be tested, accurately weigh it, add acetonitrile-water (40:60, v / v) to dissolve and quantitatively dilute to prepare 0.5 mg of parecoxib sodium per 1 mL to be tested The solution of the sample, as the solution to be tested;
[0056] (2) Take 10 μL of the solution to be tested and inject it into a liquid chromatograph, record the chromatogram, and calculate the content of impurities in each process according to the standard curve method.
[0057] The chromatographic conditions for liquid phase detection are: chromatographic column: Welch Ultimate XB-C18 (4.6×250mm, 5μm); mobile phase A: 0.01mol / L disodium hydrogen phosphate solution (adjust pH to 3.2 with phosphoric acid); mobile phase B : acetonitrile-methanol (80:20, v / v); detection wavelength: 215 nm; column tem...
Embodiment 3
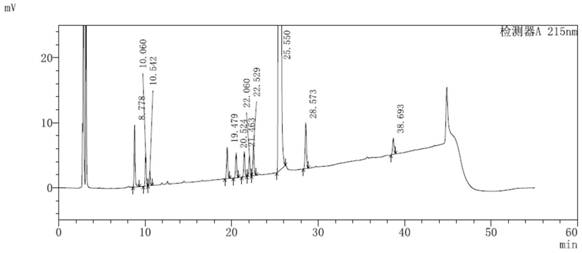
[0064] The method for detecting impurities in the parecoxib sodium synthesis process of the present embodiment adopts the following steps:
[0065] (1) Take an appropriate amount of the sample of parecoxib sodium to be tested, accurately weigh it, add acetonitrile-water (40:60, v / v) to dissolve and quantitatively dilute to prepare 0.5 mg of parecoxib sodium per 1 mL to be tested The solution of the sample, as the solution to be tested;
[0066] (2) Take 10 μL of the solution to be tested and inject it into a liquid chromatograph, record the chromatogram, and calculate the content of impurities in each process according to the standard curve method.
[0067] The chromatographic conditions for liquid phase detection are: chromatographic column: YMC-Pack ODS-AQ (4.6×250mm, 5μm); mobile phase A: 0.01mol / L disodium hydrogen phosphate solution (adjust pH to 2.8 with phosphoric acid); mobile phase B: acetonitrile-methanol (85:15, v / v); detection wavelength: 215 nm; column temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com