Negative-type photosensitive resin composition, cured film, and organic EL display and manufacturing method therefor
A photosensitive resin and composition technology, which is applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, organic chemistry, etc., can solve the problems of reduced visibility and contrast, and achieves suppression of changes in the size and width of pattern openings and light-shielding properties. Excellent, high-sensitivity results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
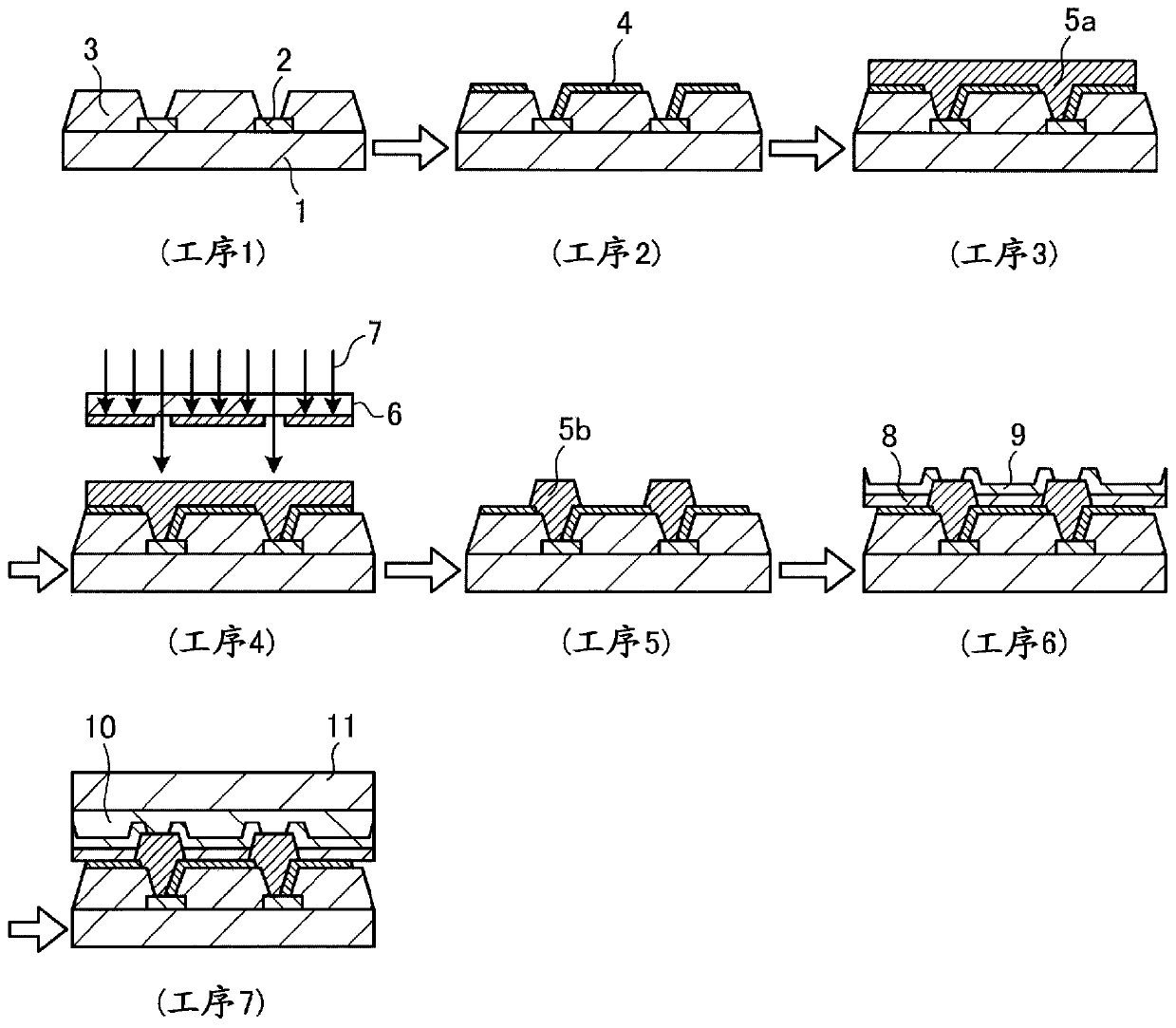
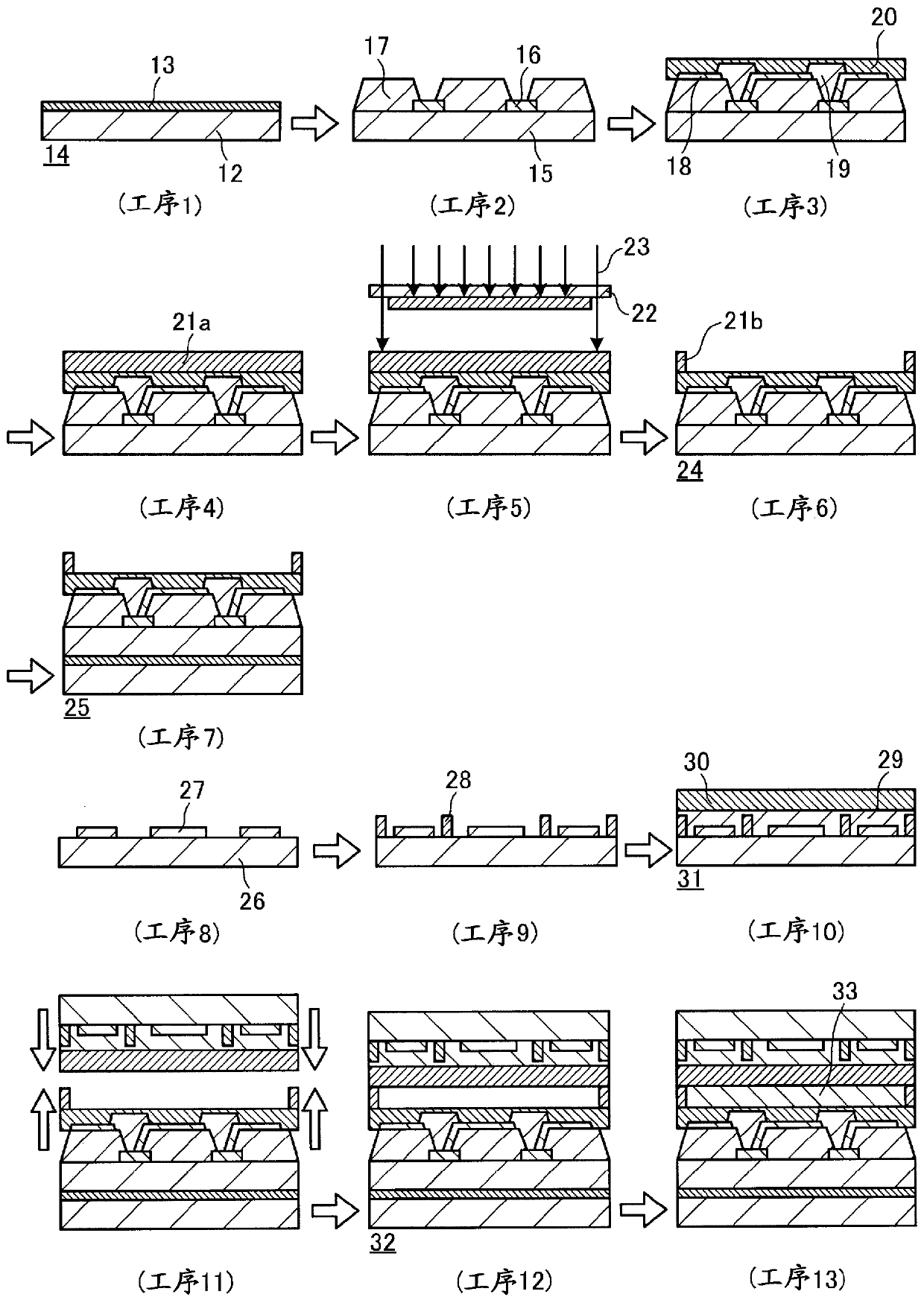
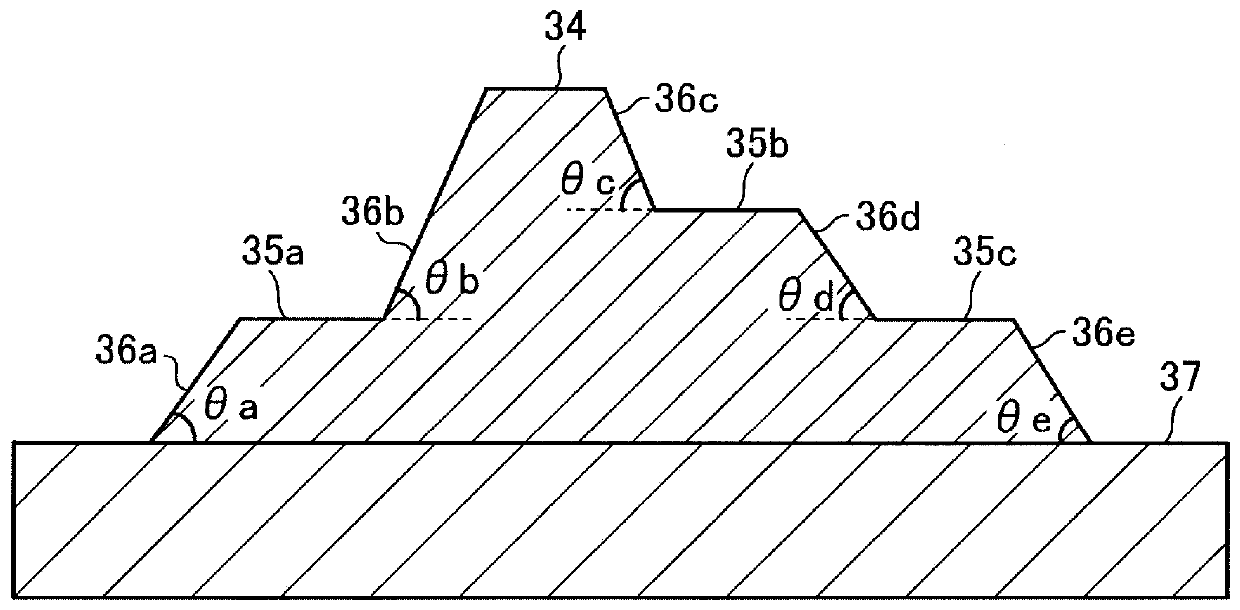
[0606] The present invention will be described more concretely below with reference to Examples and Comparative Examples, but the present invention is not limited to these ranges. In addition, among the compounds used, the compound which used the abbreviation will show the name below.
[0607] 6FDA: 2,2-(3,4-dicarboxyphenyl)hexafluoropropane dianhydride; 4,4’-hexafluoropropane-2,2-diyl-bis(1,2-phthalic anhydride)
[0608] A-BPEF: "NK ESTER" (registered trademark) A-BPEF (manufactured by Shin-Nakamura Chemical Co., Ltd.; 9,9-bis[4-(2-acryloyloxyethoxy)phenyl]fluorene)
[0609] A-DCP: "NK ESTER" (registered trademark) A-DCP (manufactured by Shin-Nakamura Chemical Co., Ltd.; dimethylol-tricyclodecane diacrylate)
[0610] A-DPH-6E: "NK ESTER" (registered trademark) A-DPH-6E (manufactured by Shin-Nakamura Chemical Co., Ltd.; ethoxylated dipentaerythritol hexaacrylate having six oxyethylene groups in the molecule)
[0611] APC: Argentum-Palladium-Cupper (silver-palladium-copper al...
Synthetic example (A
[0667]18.31 g (0.05 mol) of BAHF, 17.42 g (0.3 mol) of propylene oxide, and 100 mL of acetone were weighed and dissolved in a three-necked flask. A solution in which 20.41 g (0.11 mol) of 3-nitrobenzoyl chloride was dissolved in 10 mL of acetone was added dropwise thereto. After completion of the dropwise addition, the reaction was carried out at -15°C for 4 hours, and then returned to room temperature. The precipitated white solid was collected by filtration, and vacuum-dried at 50°C. 30 g of the obtained solid was put into a 300 mL stainless steel autoclave, dispersed in 250 mL of 2-methoxyethanol, and 2 g of 5% palladium-carbon was added. Hydrogen gas was introduced therein with a balloon, and it was made to react at room temperature for 2 hours. After 2 hours, it was confirmed that the balloon was no longer deflated. After completion|finish of reaction, it filtered and removed the palladium compound which is a catalyst, it distilled off and concentrated under reduced pr...
Synthetic example 1
[0669] The synthesis of synthetic example 1 polyimide (PI-1)
[0670] Under dry nitrogen flow, weigh BAHF 31.13g (0.085mol; relative to the structural units derived from all amines and their derivatives is 77.3mol%), SiDA 1.24g (0.0050mol; relative to the structural units derived from all The structural units of amines and their derivatives are 4.5 mol%), MAP 2.18g (0.020 mol; relative to the structural units derived from all amines and their derivatives is 18.2 mol%)), NMP 150.00g to make it dissolve. A solution in which ODPA 31.02 g (0.10 mol; 100 mol % relative to structural units derived from all carboxylic acids and derivatives thereof) was dissolved in NMP50.00 g was added thereto, stirred at 20° C. for 1 hour, and then heated at 50° C. Stirring was continued for 4 hours. Then, 15 g of xylene was added, and it stirred at 150 degreeC for 5 hours, azeotroping water and xylene. After completion of the reaction, the reaction solution was poured into 3 L of water, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| double bond equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com