Preparation method of inhalation solution for treating respiratory diseases
A technology for solution and respiratory tract, applied in the field of preparation of inhalation solution, which can solve the problems that patients are difficult to take and the scope of clinical treatment is narrow, and achieve the effect of stable quality, simple production process and good process reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Ambroxol hydrochloride: 15g
[0047] Clenbuterol hydrochloride: 0.01g
[0048] Citric acid: 2.0g
[0049] Disodium hydrogen phosphate: 7.0g
[0051] Water for injection: up to 2000mL
[0052] (1) Dissolving citric acid and disodium hydrogen phosphate in water for injection with a total amount of 70% to obtain solution I for subsequent use;
[0053] (2) Sodium chloride is dissolved in 10% water for injection to obtain solution II for subsequent use;
[0054] (3) Add ambroxol hydrochloride and clenbuterol hydrochloride to I, stir until completely dissolved to obtain solution III;
[0055] (4) Then add the sodium chloride solution II to III, make up the water for injection to the full amount, stir and mix evenly.
[0056] Adjust pH to 5.0.
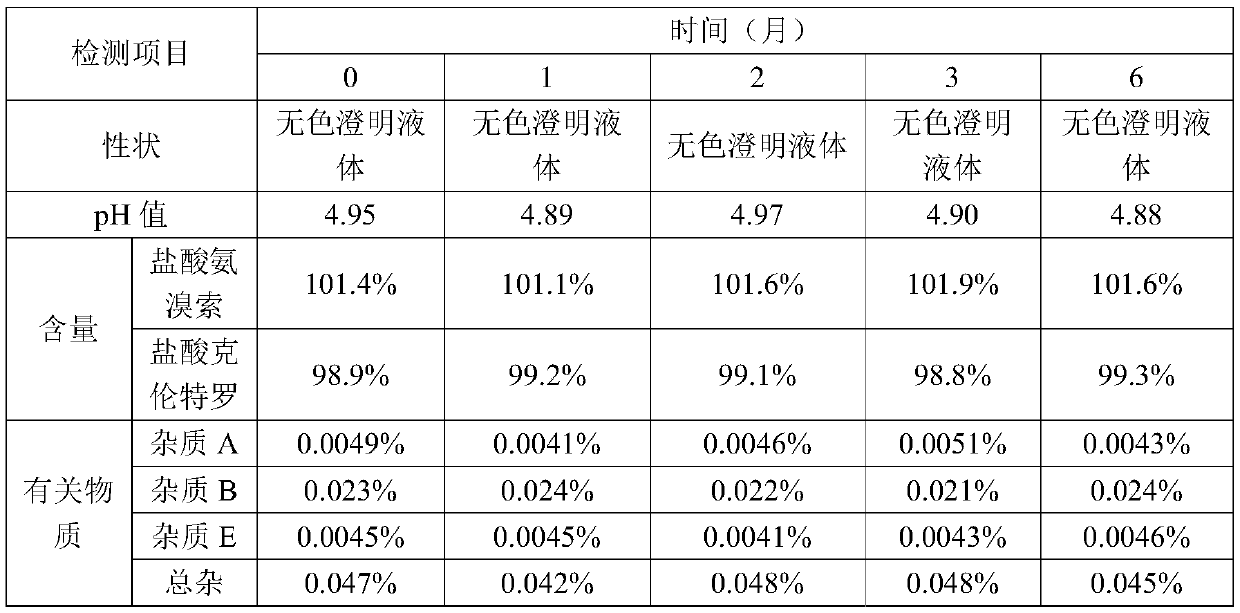
[0057] Filter with a 0.45μm filter element, and then filter with two 0.22μm filter elements; take a sample to detect the intermediate; the pH range is 4.5-5.5; the content is 97-103%.
[0058] Fill...
Embodiment 2
[0063] Ambroxol hydrochloride: 15g
[0064] Clenbuterol hydrochloride: 0.01g
[0065] Citric acid: 0.8g
[0066] Disodium hydrogen phosphate: 1.8g
[0067] Sodium chloride: 17.2g
[0068] Water for injection: up to 2000mL
[0069] (1) dissolving citric acid in water for injection of 70% of the total amount to obtain solution I for subsequent use;
[0070] (2) Sodium chloride is dissolved in 10% water for injection to obtain solution II for subsequent use;
[0071] (3) Disodium hydrogen phosphate is mixed with 500ml of 1mol / L solution to obtain solution III for subsequent use;
[0072] (4) Add ambroxol hydrochloride and clenbuterol hydrochloride to solution 1, stir until completely dissolved; then add solution II, stir, and mix well.
[0073] Use solution III to adjust the pH value to 5.0, make up the water for injection to the full amount, and mix well.
[0074] Use a 0.45μm filter element to filter, and then use two 0.22μm filter elements to filter. Sampling for detec...
Embodiment 3
[0080] Ambroxol hydrochloride: 30g
[0081] Clenbuterol hydrochloride: 0.02g
[0082] Citric acid: 2.0g
[0083] Disodium hydrogen phosphate: 7.0g
[0084] Sodium chloride: 15.0g
[0085] Water for injection: up to 2000mL
[0086] (1) dissolving citric acid and disodium hydrogen phosphate in water for injection with a total amount of 70% to obtain solution I for subsequent use;
[0087] (2) Sodium chloride is dissolved in 10% water for injection to obtain solution II for subsequent use;
[0088] (3) Add ambroxol hydrochloride and clenbuterol hydrochloride to I, stir until completely dissolved to obtain solution III;
[0089] (4) Then add the sodium chloride solution II to III, make up the water for injection to the full amount, stir and mix evenly.
[0090] Adjust pH to 5.0.
[0091] Use a 0.45μm filter element to filter, and then use two 0.22μm filter elements to filter. Sampling for detection of intermediates. The pH range is 4.5-5.5; the content is 97-103%.
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com