Method for constructing retinal pigment degeneration disease model by utilizing Gm20541 gene and application
A technology for retinal pigmentation and degenerative diseases, applied to other methods of inserting foreign genetic materials, stably introducing foreign DNA into chromosomes, recombinant DNA technology, etc., can solve the lack of RP disease models, unclear pathogenic mechanisms of RP, RP Difficulties in clinical diagnosis and treatment of diseases, etc., to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
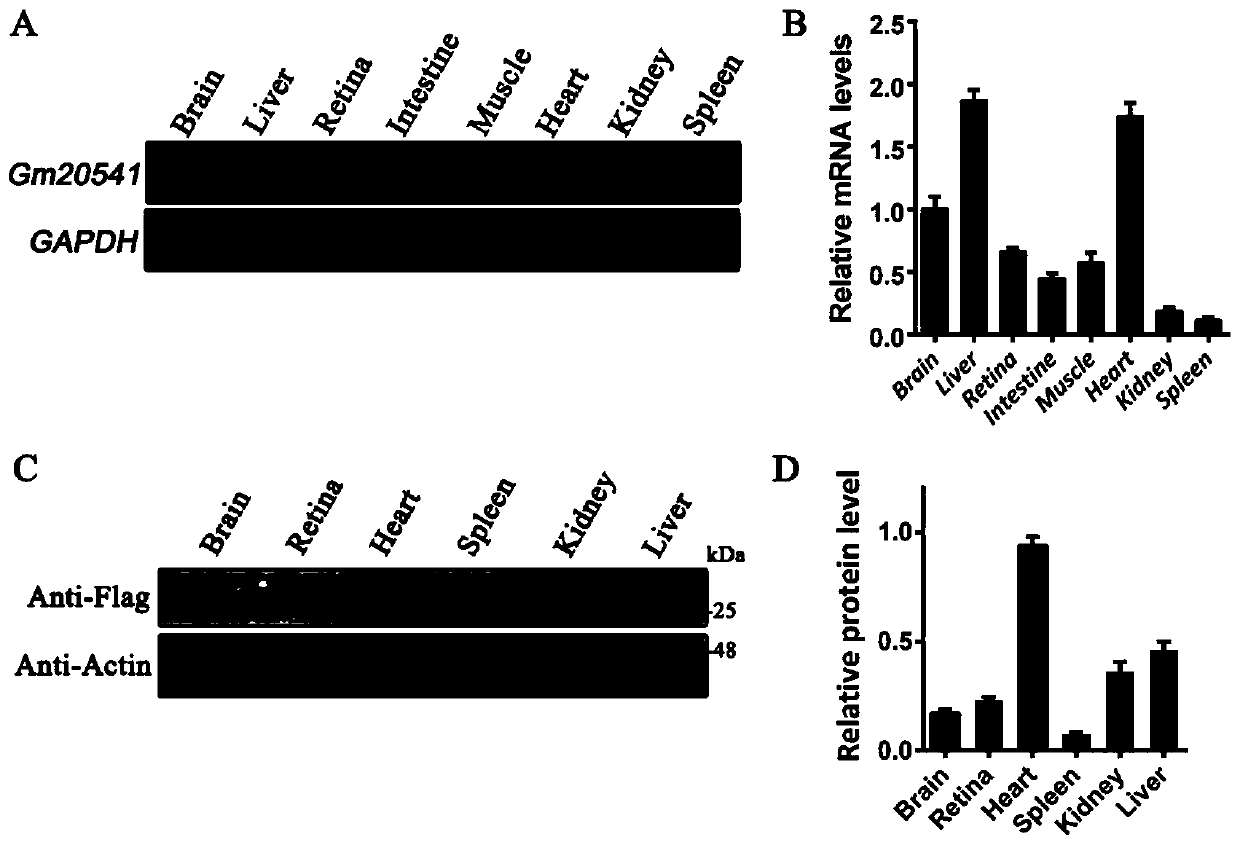
[0052] 1 RT-PCR method was used to detect the expression of Gm20541 gene in different tissues and organs.
[0053] Methods: Total RNA was extracted from mouse brain, liver, retina, intestine, muscle, heart, kidney and spleen, and cDNA was synthesized with a cDNA synthesis kit (Invitrogen, Waltham, MA, USA). Primers were designed according to the cDNA sequence of Gm20541:
[0054] Gm20541-cDNA-F: 5'-TCGGTCTCATCTTCATTCCC-3';
[0055] Gm20541-cDNA-R: 5'-GGAAGGCTCTGTTCCGGTAT-3'.
[0056] Using the extracted cDNA as a template, RT-PCR was performed. After amplification, electrophoresis was carried out, and the results were shown in figure 1 .
[0057] see results figure 1 In A and B, it can be seen that the expression of Gm20541 gene in mouse brain, liver, retina, intestine, muscle, heart, kidney and spleen was detected by RT-PCR method, and it was found that in these tissues and organs, Gm20541 was expressed expression, indicating that the gene may play an important role in ...
Embodiment 2
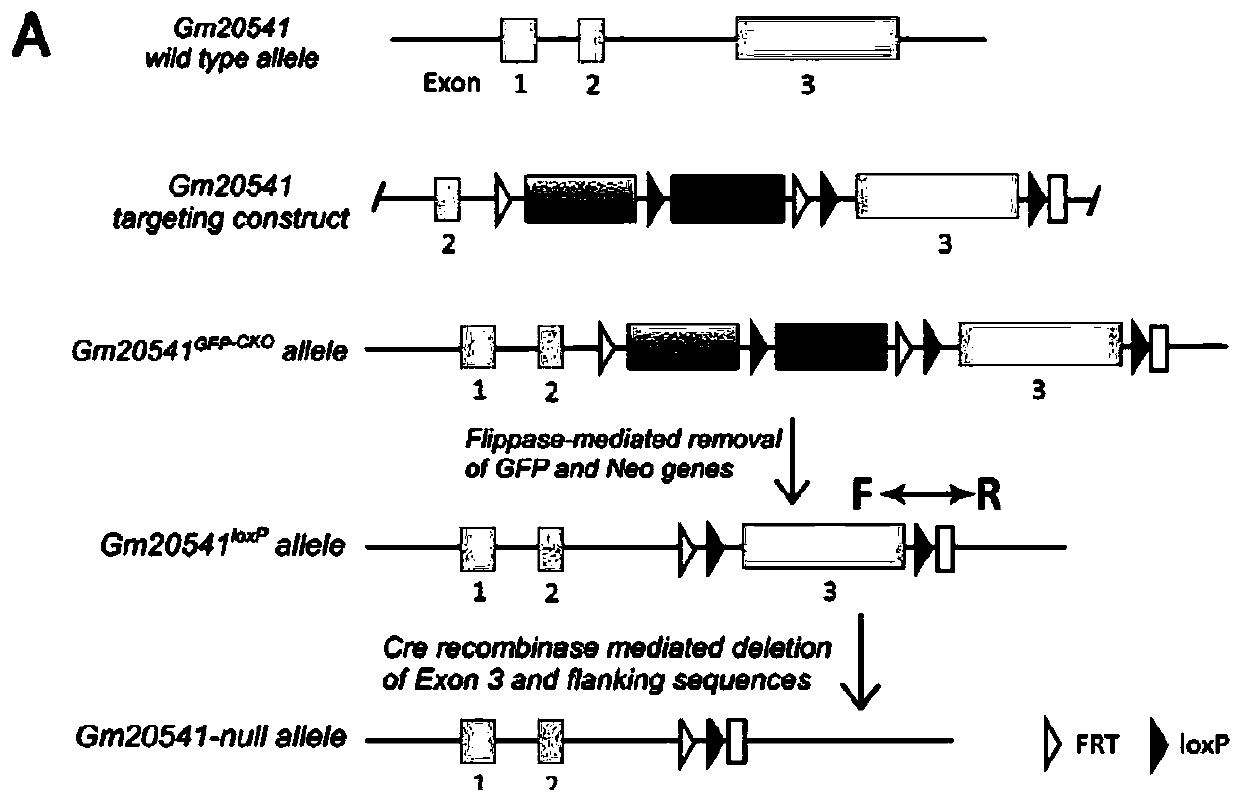
[0071] In this example, mouse is used as the target animal, and the method for constructing the retinitis pigmentosa disease model provided by the present invention is described. The route of Gm20541 gene knockout is as follows: figure 2 As shown, the specific operation is as follows:
[0072] Steps for obtaining knockout mice:
[0073] 1 The 5' arm homologous to the mouse Gm20541 gene, the expression cassette containing the reporter gene GFP, the expression cassette with the NEO resistance gene, the third exon and the 3' end of the loxP site arranged in the same direction at both ends Arm cloned into BAC vector ( figure 2 ) to replace the third exon of the Gm20541 gene to be knocked out;
[0074] 2 Using DNA homologous recombination technology to replace the third exon in the Gm20541 gene to obtain mouse embryonic stem cells with conditional knockout of the Gm20541 gene;
[0075] 3 Using the embryonic stem cells obtained in step 2 to prepare chimeric mice containing Gm20...
Embodiment 3
[0121] ERG visual acuity detection of 4-month-old Gm20541 gene knockout homozygous mice:
[0122] 1 Dark-adapted animals should be dark-adapted all night, and the environment should have absolutely no light;
[0123] Anesthesia on the 2nd day: weighing, intraperitoneal injection; deep anesthesia is appropriate;
[0124] 3 Animal fixation and mydriasis: After anesthesia is completed, the mice are fixed with adhesive tape in front of the animal test platform under dark red light illumination: it is necessary to ensure that the mice are "lying", that is, relative to the stimulation port of the flash stimulator, both eyes Highly consistent, fully exposed, drop mydriatic agent.
[0125] 4 Electrode installation: After preheating the electroretinograph (Espion Visual Electrophysiology System, Diagnosys LLC, Littleton, MA, USA), apply conductive paste to the electrode of the ear clip, clip the tail, and insert it into the "ground" interface of the amplifier; the double-ended needle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com