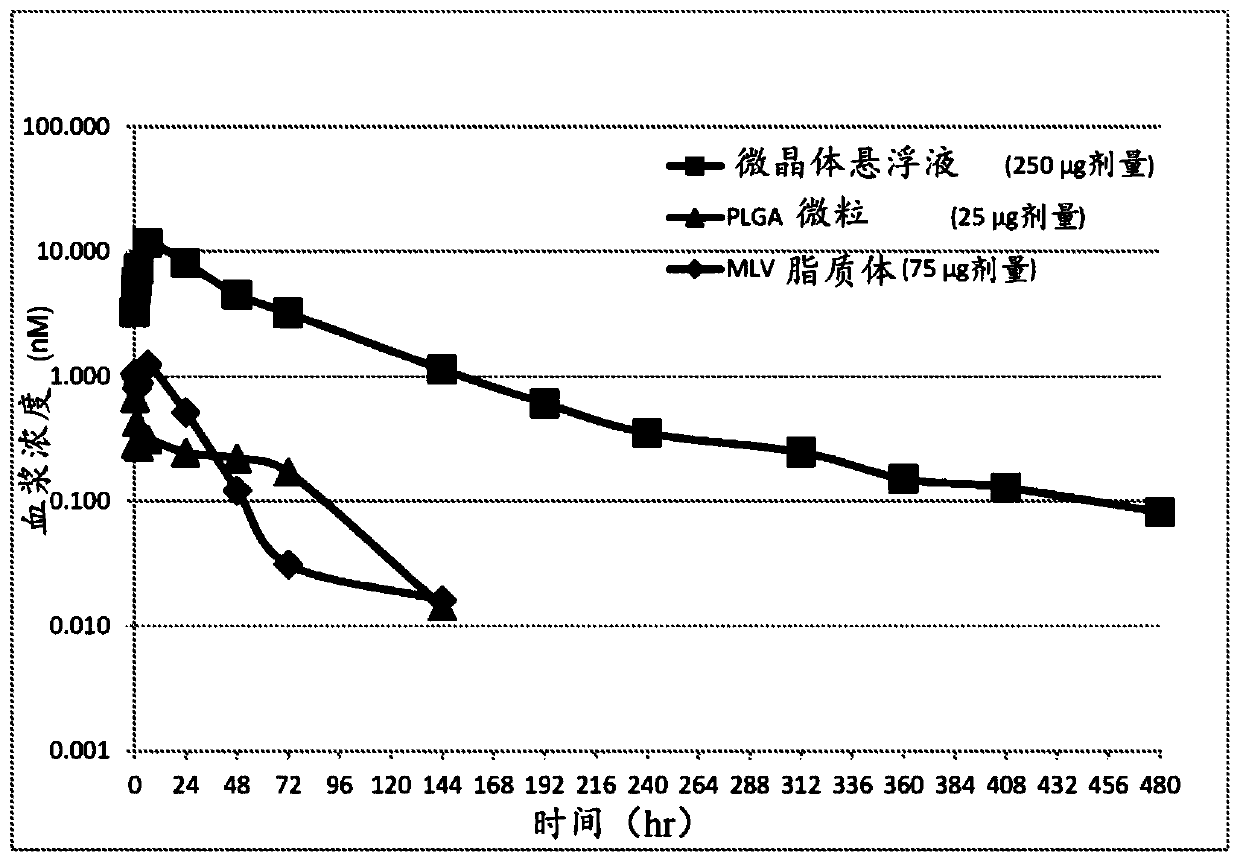
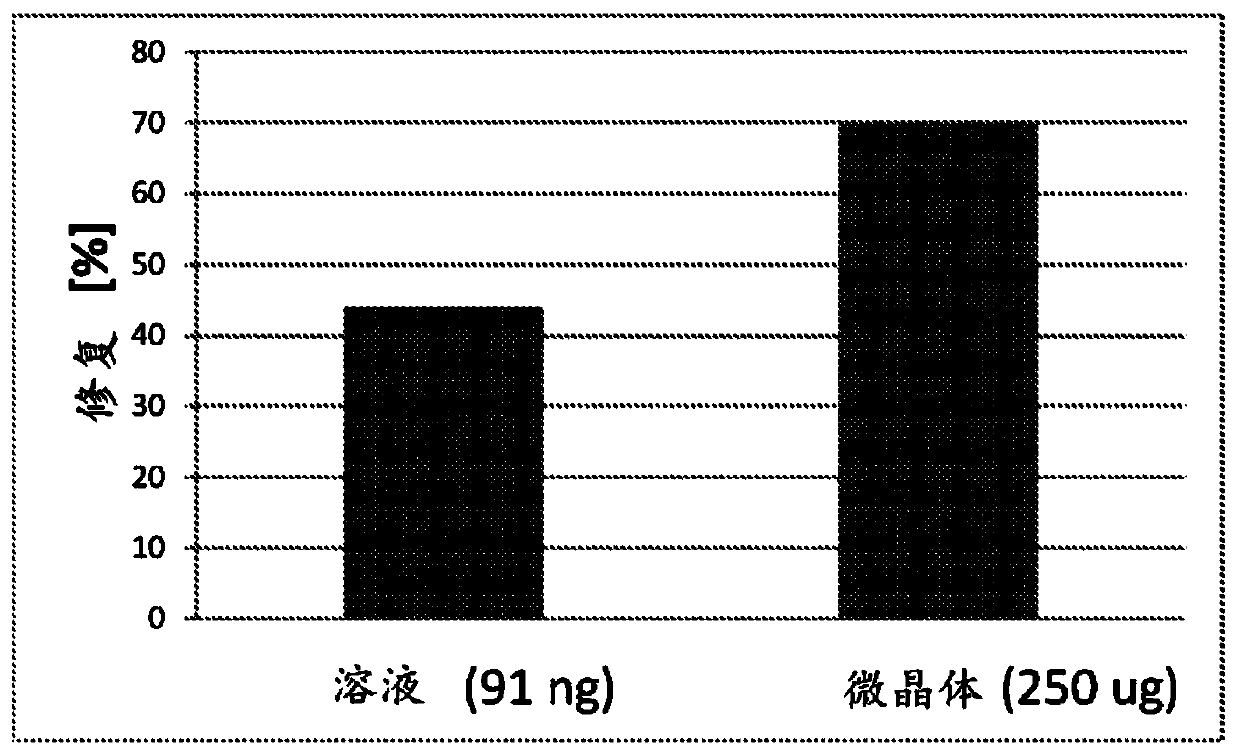
Extended release formulations for intra-articular applications
A composition and drug technology, applied in the field of extended release formulations, can solve the problems of frequent injection, short synovial residence half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0131] Example 1. Preparation of Microcrystals of Compound A
[0132] The production batch of Compound A was observed under an electron microscope ( figure 1 ). The particles are irregular and columnar and range in length from about 100 nm up to 140 μm, but most particles are smaller than 50 μm. These particles have a tendency to agglomerate.
[0133] Compound A can be micronized using any method known to those skilled in the art. The unmilled compound A was subjected to a size reduction process to obtain particle size. The size reduction of Compound A can be performed using any known method, such as by jet milling techniques, and more specifically, by spiral jet milling techniques, fluidized bed opposed jet milling techniques, or annular jet milling techniques.
[0134] In one embodiment, the particle size distribution of Compound A is D90≦50 μm. In another embodiment, Compound A has a particle size distribution of D90 < 30 μm, < 25 μm, < 20 μm or < 15 μm.
[0135] In...
example 2
[0138] Example 2. Formulation Screening with PVP-K12 or CMC
[0139] A microcrystalline suspension of Compound A prepared in Example 1 was prepared with a solution containing PVP-K12 or carboxymethylcellulose (CMC) sodium salt. The resuspension of the formulation was tested by repeated centrifugation and shaking resuspension. Formulations containing CMC were not resuspended after centrifugation. Formulations containing PVP-K12 were further investigated.
example 3
[0140] Example 3. Formulations P1-P8 (PVP-K12 with F68, F127, SDS or egg PC)
[0141] Placebo solutions P1 to P8 were prepared according to the ingredients (w / v %) listed in Table 2.
[0142] Table 2
[0143] Preparations PVP-K12(%) additional excipients Additional excipients (%) P1 2% F68 0.2% P2 2% F68 0.5% P3 2% F127 0.2% P4 2% F127 0.5% P5 2% SDS 0.05% P6 2% SDS 0.01% P7 2% Egg PC 0.2% P8 2% Egg PC 0.5%
[0144] Formulations were prepared in R2 vials containing approximately 5 mg of Compound A microcrystals and filled with placebo solutions P1 to P8 to achieve a drug substance concentration of 5 mg / mL. The formulations were tested for resuspension and sedimentation rate according to the sequential steps listed in column 1 of Table 3. After each step, the turbidity of the samples was observed and documented by photographs and is summarized in Table 3.
[0145] table 3
[0146]
[0147] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com