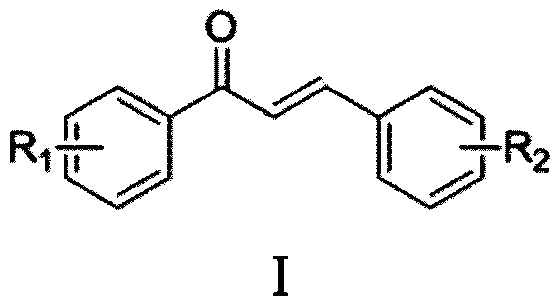
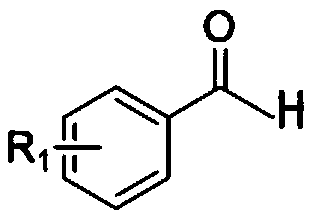
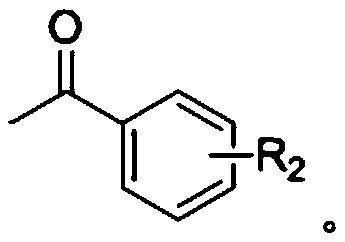
Synthesis method of chalcone derivative
A technology for chalcones and a synthesis method is applied in the field of synthesis of chalcone derivatives and achieves the effects of low price, easy operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: A kind of synthetic method of chalcone derivatives:
[0046] Add polyphosphoric acid PPA (analytical pure) 5mmol and solvent 1,4-dioxane (analytical pure) 2mL in the round bottom flask, then add 98% concentrated sulfuric acid 20mmol, benzaldehyde 0.7mmol, p-methyl (p -CH 3 ) 1 mmol of acetophenone, heated and stirred in an oil bath at 90°C under nitrogen protection for 2 hours; after the reaction was completed, extracted 3 times with 50 ml of ethyl acetate, took the organic layer, washed with tap water and saturated sodium chloride solution in turn, and then washed with anhydrous sulfuric acid The organic layer was dried and washed with sodium, and filtered; the obtained filtrate was concentrated under reduced pressure, and the concentrated residue was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate volume ratio: 50:1) to obtain chalcones derivatives Material I-1; Yield 95%.
[0047] The nuclear magnetic resonan...
Embodiment 2
[0048] Embodiment 2: A kind of synthetic method of chalcone derivatives:
[0049] Add 5mmol of polyphosphoric acid PPA and 2-3mL of solvent 1,4-dioxane into the flask, then add 20mmol of 98% concentrated sulfuric acid, 0.7mmol of benzaldehyde, 2,4-chlorinated (m,m-Cl 2 ) 1 mmol of acetophenone, heated and stirred at 90°C for 2 h under nitrogen protection; after the reaction, extracted 3 times with 50 ml of ethyl acetate, took the organic layer, washed with distilled water and saturated sodium chloride solution successively, and dried with anhydrous sodium sulfate The organic layer was washed and filtered; the obtained filtrate was concentrated under reduced pressure, and the concentrated residue was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate volume ratio: 40:1) to obtain chalcone derivative I -2; Yield 75%.
[0050] The nuclear magnetic resonance (NMR) of the prepared chalcone derivatives (I-2) 1 H NMR and 13 C NMR) detection ...
Embodiment 3
[0051] Embodiment 3: A kind of synthesis method of chalcone derivatives:
[0052] Add 5 mmol of polyphosphoric acid PPA and 2 mL of solvent 1,4-dioxane to the flask, then add 20 mmol of 98% concentrated sulfuric acid, 0.7 mmol of benzaldehyde, and 1 mmol of p-bromo (p-Br) acetophenone in sequence, Heat and stir in an oil bath at 90°C for 2 hours; after the reaction, extract 3 times with 50 ml of ethyl acetate, take the organic layer, wash it with water and saturated sodium chloride solution in sequence, then dry the washed organic layer with anhydrous sodium sulfate, and filter; The obtained filtrate was concentrated under reduced pressure, and the concentrated residue was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate volume ratio: 30:1) to obtain chalcone derivative I-3; the yield was 80% .
[0053] The nuclear magnetic resonance (NMR) of the prepared chalcone derivatives (I-3) 1 H NMR and 13 C NMR) detection data are: 1 HNMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com