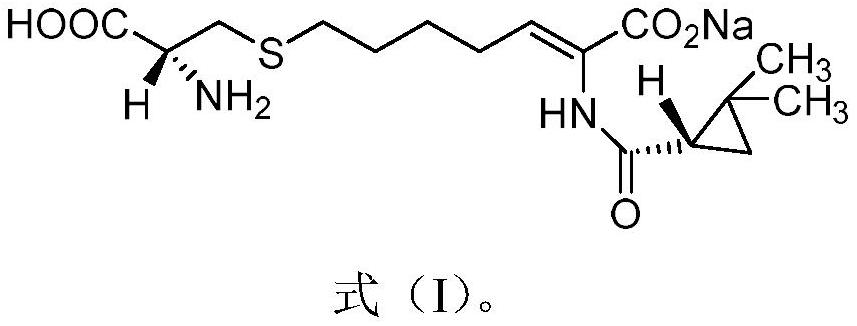
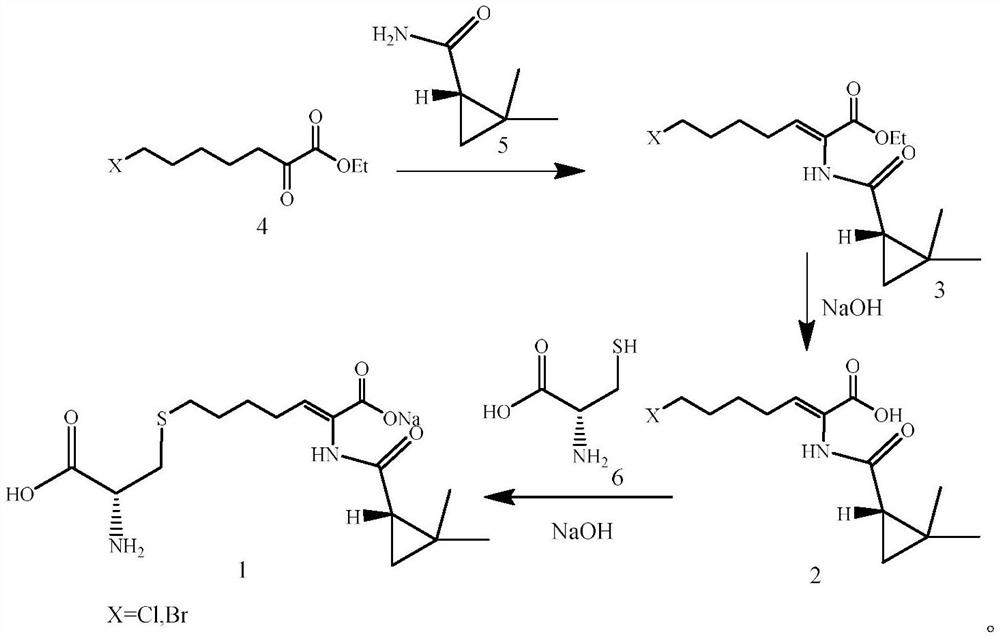
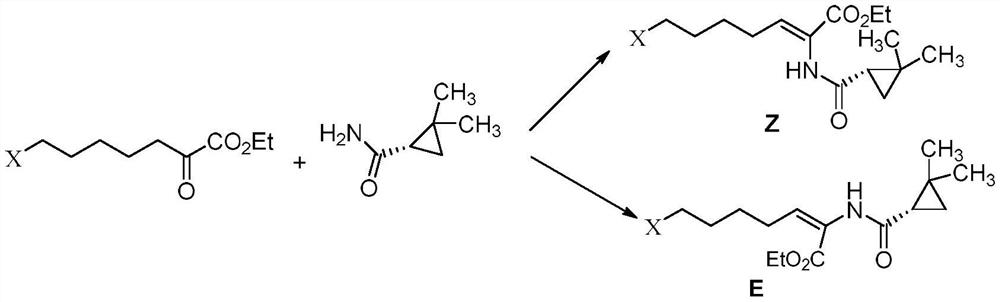
A kind of preparation method of key intermediate of cilastatin sodium
A technology of cilastatin sodium and intermediates, applied in the field of organic chemical synthesis, can solve the problems of difficult removal of impurities, low conversion rate, easy generation of impurities, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step (1), in a reaction device equipped with a water separator, 50g (0.24mol) of 7-chloro-2-oxoheptanoic acid ethyl ester, (S)-2, 2-dimethylcyclopropanecarboxamide 27.16 g (0.24 mol) and 0.9 g of p-toluenesulfonic acid were added to 240 mL of toluene, and the mixture was kept under reflux for 10 h. After the reaction, the reaction solution was cooled to room temperature. The HPLC purity was 89.5%, and the E-configuration impurity was 10%.
[0039] Step (2), the reaction solution obtained in the previous step is placed in a 30°C incubator, and irradiated with ultraviolet light under a 20W ultraviolet lamp (the emission wavelength is 280 nm, and the control light intensity is 450 μW cm -2 ), after irradiating for 60 min, the reaction solution was washed twice with water, the organic layer was dried over anhydrous sodium sulfate, filtered, and the toluene was evaporated to obtain (Z) 7-chloro-2((S)-2,2-dimethyl ring Propanecarboxamido)-2-heptenoic acid ethyl ester, molar y...
Embodiment 2
[0041] Step (1), in a reaction device equipped with a water separator, 50g (0.24mol) of 7-chloro-2-oxoheptanoic acid ethyl ester, (S)-2, 2-dimethylcyclopropanecarboxamide 27.16 g (0.24 mol) and 0.9 g of p-toluenesulfonic acid were added to 240 mL of benzene, and the mixture was kept under reflux for 13 h. After the reaction, the reaction solution was cooled to room temperature. The HPLC purity was 87.3%, and the E-configuration impurity was 12%.
[0042] Step (2), the reaction solution obtained in the previous step was placed in a 30°C incubator, and irradiated with ultraviolet light under a 20W ultraviolet lamp (the emission wavelength was 300 nm, and the control light intensity was 450 μW cm -2 ), after irradiating for 60 min, the reaction solution was washed twice with water, the organic layer was dried over anhydrous sodium sulfate, filtered, and the benzene was evaporated to obtain (Z) 7-chloro-2((S)-2,2-dimethyl ring Propanecarboxamido)-2-heptenoic acid ethyl ester, mola...
Embodiment 3
[0044] Step (1), in a reaction device equipped with a water separator, 50g (0.24mol) of 7-chloro-2-oxoheptanoic acid ethyl ester, (S)-2, 2-dimethylcyclopropanecarboxamide 41.74 g (0.36 mol) and 0.9 g of p-toluenesulfonic acid were added to 240 mL of xylene, and the mixture was kept under reflux for 10 h. After the reaction was completed, the reaction solution was cooled to room temperature. The HPLC purity was 88.0%, and the E-configuration impurity was 9%.
[0045] Step (2), the reaction solution obtained in the previous step was placed in a 40°C incubator, and irradiated with ultraviolet light under a 20W ultraviolet lamp (the emission wavelength was 250 nm, and the control light intensity was 450 μW cm -2 ), after irradiating for 60 min, the reaction solution was washed twice with water, the organic layer was dried over anhydrous sodium sulfate, filtered, and the xylene was evaporated to obtain (Z) 7-chloro-2((S)-2,2-dimethylene) Cyclopropanecarboxamido)-2-heptenoic acid et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com