Dimethylaminomicheliolide fumarate crystal form C and preparation method thereof
A technology of fumarate and dimethylamine, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of small particle size, easy aggregation, and small bulk density of crystal form A, and achieve high product yield and repeatability Good performance and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
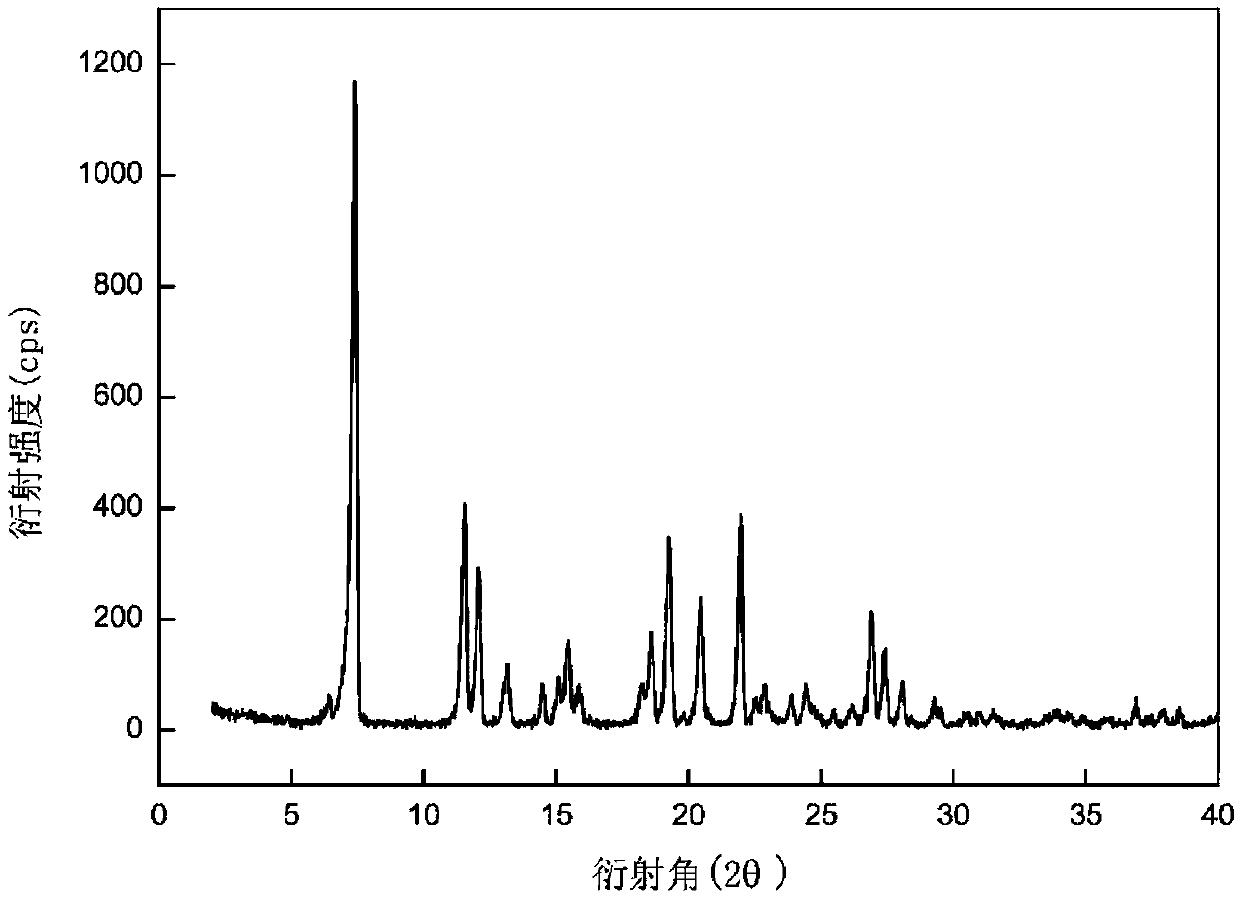
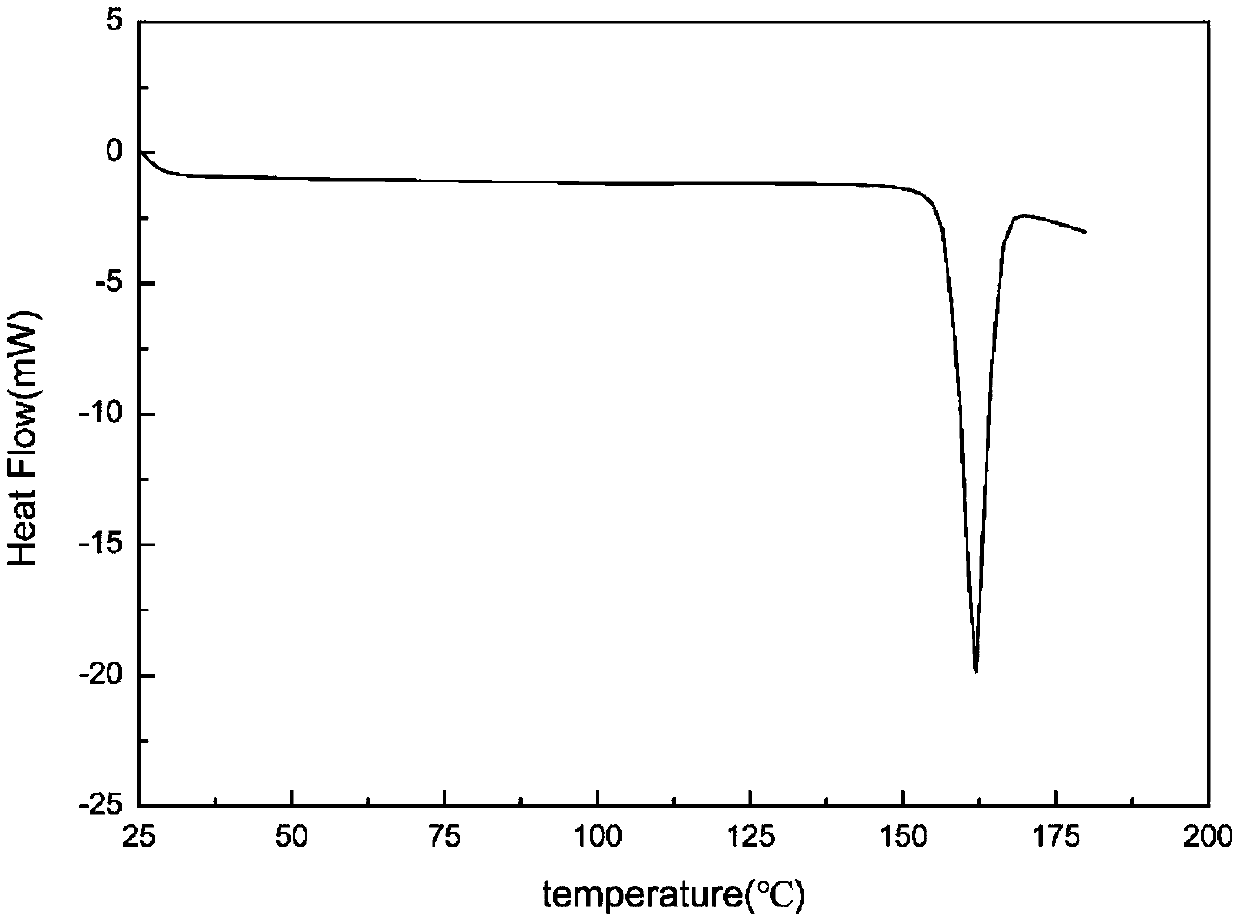
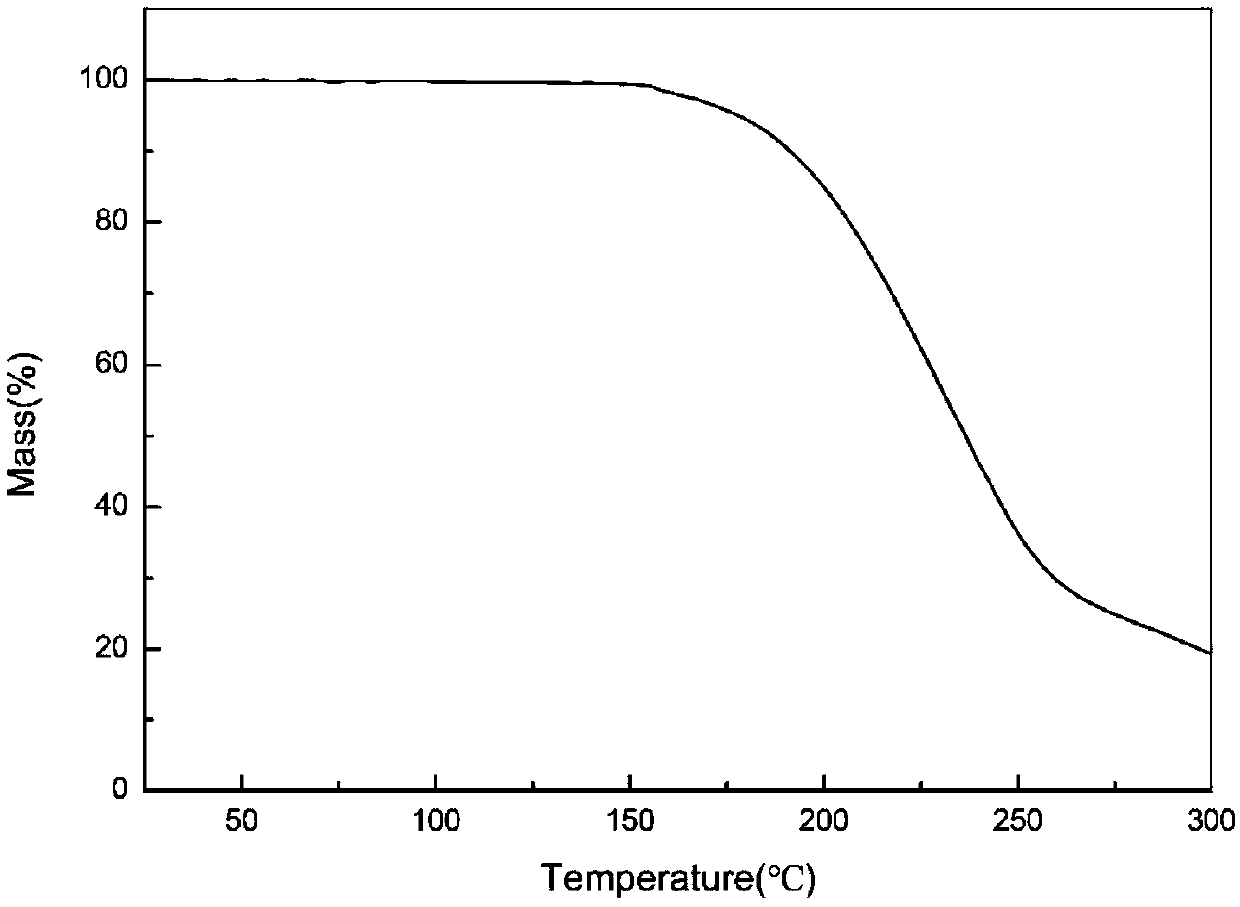
[0029]Put 0.03g of dimethylamine michelolactone fumarate raw material in a 4mL sample bottle, add 3g of acetone solvent to make it dissolved and in a supersaturated state, stir at 35°C, and perform solvent-mediated crystallization. After 24h The suspension was filtered to obtain a white solid, which was dried under normal pressure at 30°C for 6 hours to obtain crystals of dimethylaminomildolide fumarate. The X-ray powder diffraction pattern of the product is represented by 2θ at 6.4°, 7.4°, 11.6°, 12.1°, 13.1°, 14.5°, 15.1°, 15.5°, 15.9°, 18.3°, 18.6°, 19.3°, 20.5° , 22.0°, 26.9°, and 27.4° have characteristic peaks, of which 6.4° is the initial peak, and the relative intensity of the characteristic peak at 7.4° is 100%. In addition, the X-ray powder diffraction pattern of the product is represented by 2θ angle Angle also has characteristic peaks at 19.8°, 22.5°, 22.9°, 23.9°, 24.4°, 25.5°, 26.2°, 28.1°, 29.3°, 29.5°, 30.6°, 31.0°, 31.5°, and figure 1 unanimous. The DSC spec...
Embodiment 2
[0031] Put 3g of dimethylamine michelolactone fumarate raw material in a 150mL crystallizer, add 100g of acetonitrile solvent to dissolve it and make it in a supersaturated state, stir at 50°C, and perform solvent-mediated crystallization. After 48h, the The suspension was filtered to obtain a white solid, which was dried under normal pressure at 40°C for 10 h to obtain crystals of dimethylamine michelactone fumarate. The X-ray powder diffraction pattern of the product is represented by 2θ, at 6.6°, 7.6°, 11.8°, 12.3°, 13.3°, 14.7°, 15.3°, 15.6°, 16.0°, 18.5°, 18.8°, 19.5°, 20.7 °, 22.2°, 27.1°, and 27.6° have characteristic peaks, of which 6.6° is the initial peak, and the relative intensity of the characteristic peak at 7.6° is 100%. In addition, the X-ray powder diffraction pattern of the product is represented by 2θ angle Diffraction angles also have characteristic peaks at 20.0°, 22.7°, 23.1°, 24.1°, 24.6°, 25.7°, 26.4°, 28.3°, 29.5°, 30.7°, 31.2°, 31.7°, and figure 1 un...
Embodiment 3
[0033] Put 0.2g of dimethylamine michelolactone fumarate raw material in a 20mL sample bottle, add 10g of tetrahydrofuran solvent to dissolve and make it in a supersaturated state, stir at 45°C, and perform solvent-mediated crystallization. After 30h The suspension was filtered to obtain a white solid, which was dried under normal pressure at 35°C for 8 hours to obtain crystals of dimethylaminomildolactone fumarate. The X-ray powder diffraction pattern of the product is represented by 2θ, at 6.3°, 7.3°, 11.4°, 12.0°, 13.0°, 14.4°, 15.0°, 15.4°, 15.8°, 18.1°, 18.5°, 19.2°, 20.4 °, 21.9°, 26.9°, and 27.3° have characteristic peaks, of which 6.3° is the initial peak, and the relative intensity of the characteristic peak at 7.3° is 100%. In addition, the X-ray powder diffraction pattern of the product is represented by 2θ angle Diffraction angles also have characteristic peaks at 19.8°, 22.5°, 22.8°, 23.8°, 24.4°, 25.5°, 26.2°, 28.0°, 29.2°, 29.5°, 30.6°, 31.0°, 31.5°, and figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com