Mimetic peptide combined with cN-II and pharmaceutical use of mimetic peptide
A technology that mimics peptides and medicinal salts, applied in the field of biotechnology medicine, can solve problems such as drug resistance, drug resistance and recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
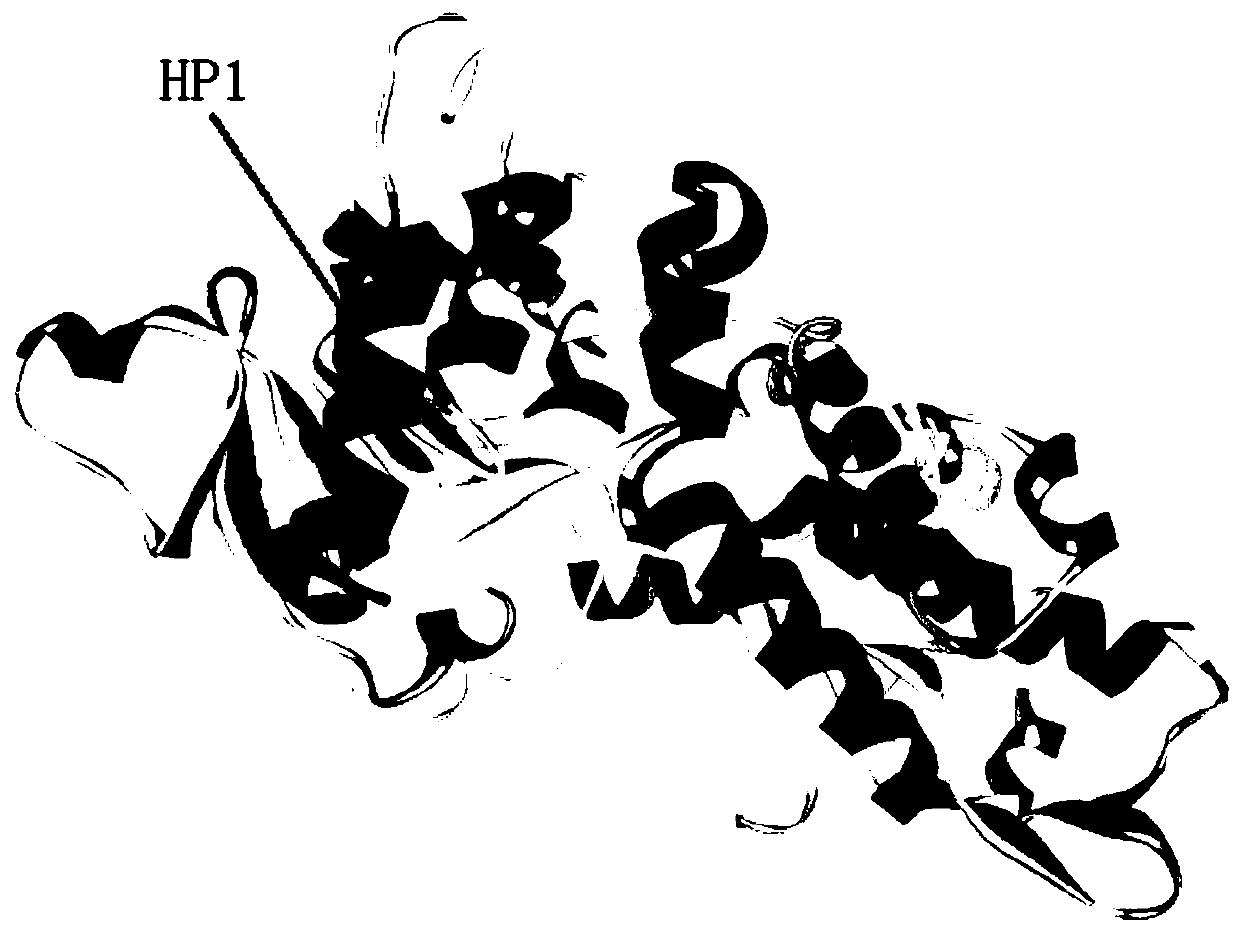
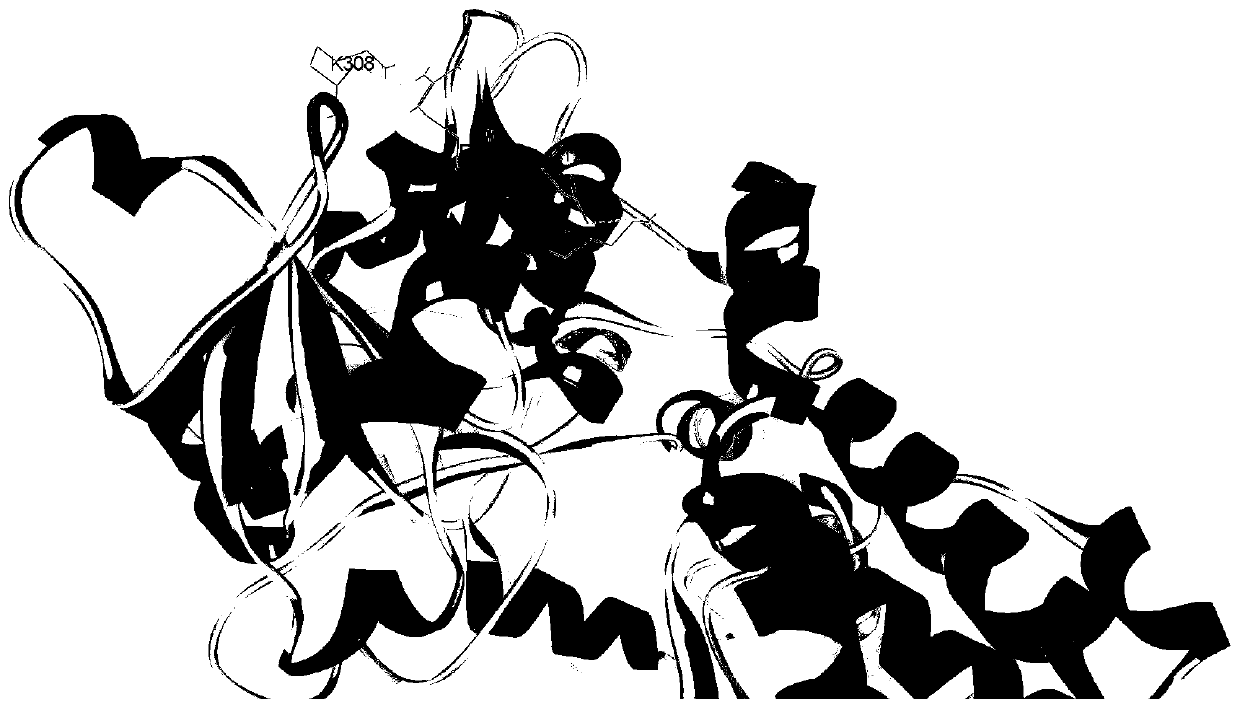
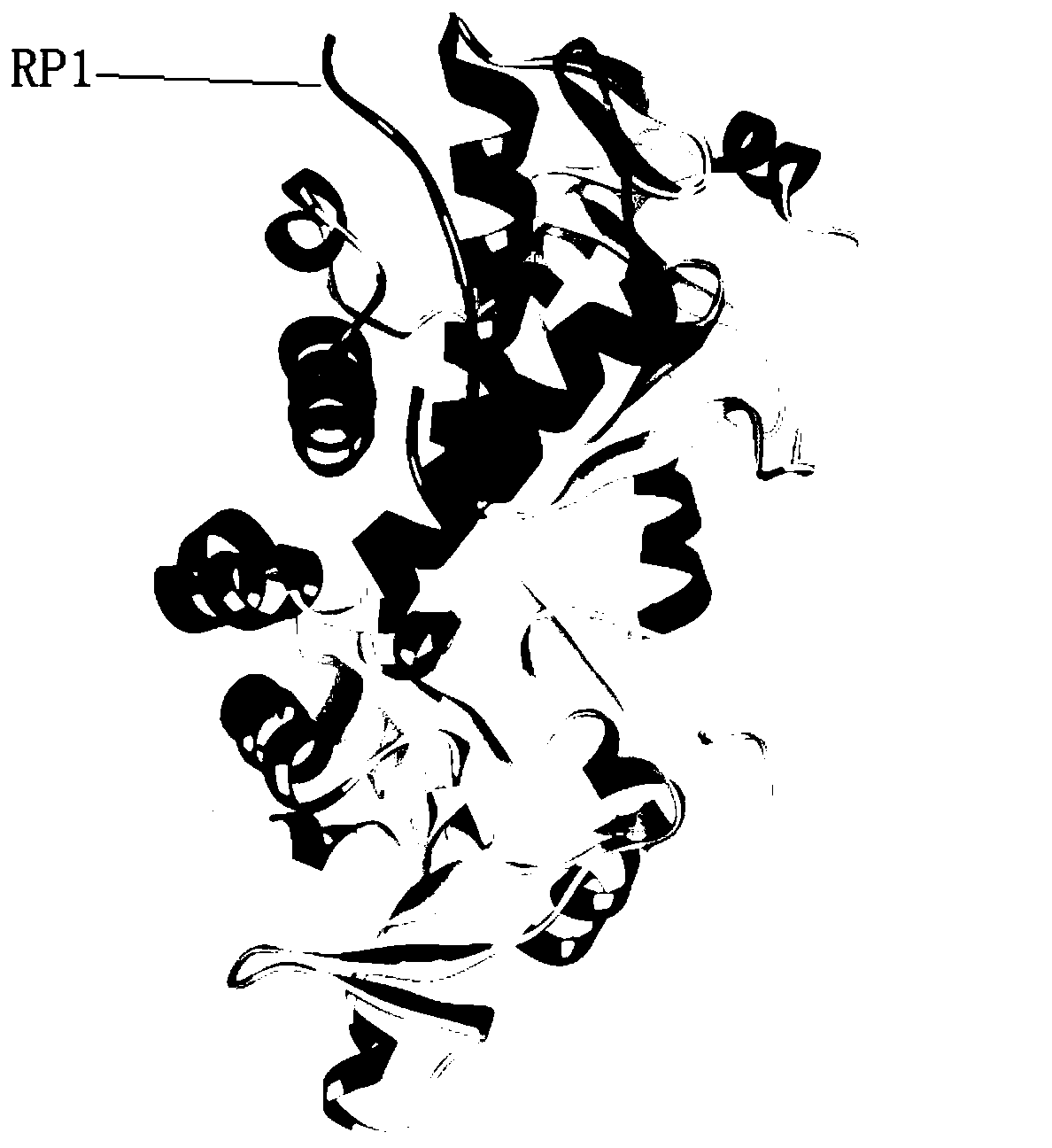
[0028] cN-II (cytoplasmic-5'-nucleotidase-II) forms a tetramer with two homologous identical dimers to play a biological role in vivo. A large number of mimetic peptides (cN-II mimeticpeptide, cNMP) that can bind to cN-II were screened by ribosome display technology; the crystal structure of cN-II was analyzed, and through repeated homology modeling, and ZDOCK of discovery studioTM , RDOCK tools for molecular docking and other bioinformatics analysis of the mimic peptides; further in vitro tests were performed to optimize the mimic peptides with obvious inhibitory effect on the expression of leukemia cell cN-Ⅱ, thus obtaining the specific binding simulation of cN-Ⅱ Peptide, which has cN-Ⅱ antagonistic effect and reverses the effect of nucleoside analog drug resistance in ALL cells, it can act on cN-Ⅱ dimer, can exert inhibitory effect on dimer formation, or act on already formed dimer or tetramer inhibits tetramer formation and its function. Figure 5 It is the dimer structur...
Embodiment 2
[0035] The preparation of the polypeptide described in the present invention can be obtained by DNA recombination technology, and can also be synthesized by chemical synthesis, such as by using Fmoc solid-phase polypeptide synthesis method. Using Wang resin as a solid-phase reaction substrate, the -COOH of the C-terminal amino acid of the target peptide chain is connected to the solid-phase resin with a coupling reagent, and then its -NH is removed 2 The above protecting group then reacts with the -COOH of the next amino acid to form a peptide bond, and this repeated operation can grow the peptide chain, that is, coupling reaction, washing, removing the amino protecting group, washing, and a new round of coupling, until Synthesize the target peptide, and finally cut the target peptide from the solid phase carrier with a cleavage reagent, and then obtain the target peptide after some processing.
[0036] The specific steps of the Fmoc solid-phase peptide synthesis method:
[0...
Embodiment 3
[0062] Cytarabine (Ara-C) belongs to cytarabine (Ara-C) is a pyrimidine anti-metabolite drug, mainly used for the treatment of acute leukemia and malignant lymphoma, especially for acute myeloid leukemia. Well, according to relevant reports: the rate of complete remission after treatment with Ara-C-containing chemotherapy regimens in patients with newly diagnosed acute myeloid leukemia is 65% to 80%. However, most patients who achieve complete remission will relapse within two years after the diagnosis, which may be related to the resistance of tumor cells to drugs, resulting in poor curative effect of subsequent treatment. After Ara-C enters tumor cells, it undergoes a two-step phosphorylation and activation reaction to generate the active product Ara-CTP, which inhibits DNA synthesis. Similarly, cN-II can also hydrolyze Ara-CMP, the monophosphate activation product of Ara-C, and inhibit its continued phosphorylation and activation, resulting in drug failure.
[0063] 6-MP (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com