Process for the preparation of thiamethoxam
A technology for thiamethoxam and its use, applied in the field of 3--5-methyl-1, capable of solving problems such as unsatisfactory yield and purity level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the synthesis of thiamethoxam
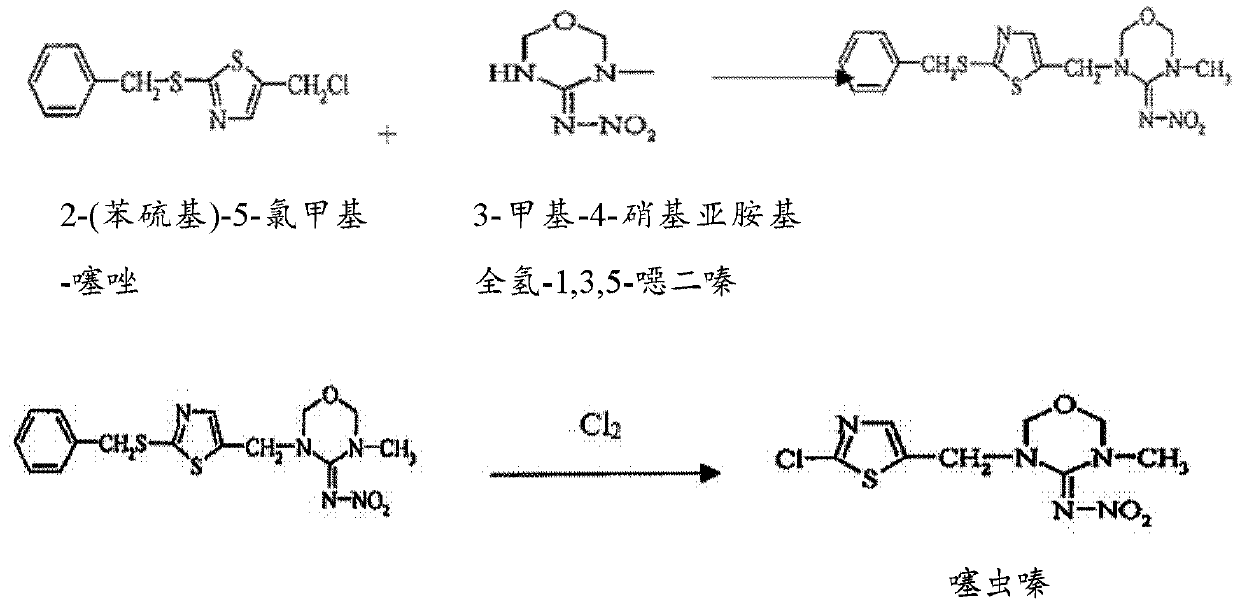
[0080] 1.1 Preparation of 3,6-dihydro-3-methyl-N-nitro-2H-1,3,5,oxadiazin-4-amine
[0081] 3,6-Dihydro-3-methyl-N-nitro-2H-1,3,5,oxadiazin-4-amine was prepared using the following reaction scheme:
[0082]
[0083]100 kg of N-methyl-nitroguanidine and 64 kg of paraformaldehyde were charged into a 1000 L reactor. 350 kg of acetic acid were added, after which the resulting mixture was heated to 70° C. and kept at this temperature for 6 hours. Thereafter, the solvent (acetic acid) was removed by distillation under vacuum. 175 kg of 10% aqueous NaOH were added with stirring, after which the resulting mixture was cooled and stirred for 30 minutes. The resulting mixture was discharged into a centrifuge for separation. The resulting cake was dried using a Biconical dryer to obtain 98 kg of 3-methyl-N-nitro-1,3,5,oxadiazine-4-imine (purity 97%, Yield 71.5%).
[0084] 1.2 Preparation of 2-chloro-allylthioisocyanide
[0...
Embodiment 2
[0096] Example 2: Preparation of Crystalline Thiamethoxam Using Methanol
[0097] 2 g of thiamethoxam prepared as in Example 1 were heated in 10 g of methanol until complete dissolution was achieved. The resulting solution was heated at reflux for 30 to 60 minutes, then cooled to room temperature. The resulting mixture was filtered to separate solids. The resulting solid was washed several times with methanol and dried under high vacuum to give technical grade pure thiamethoxam crystals (purity: 98%).
[0098] The crystals were characterized as thiamethoxam polymorph A using both infrared (IR) spectroscopy and X-ray diffraction.
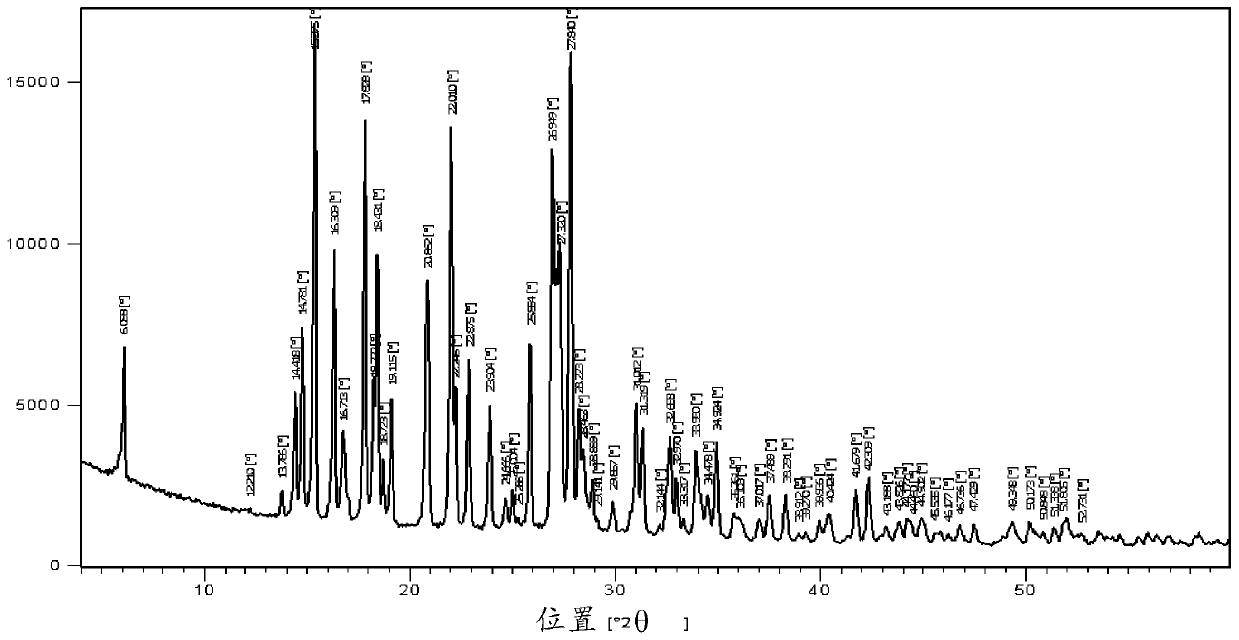
[0099] Form A thiamethoxam has figure 1 The X-ray powder diffraction pattern shown, where the reflections are listed in Table 1 below.
[0100] Table 1
[0101]
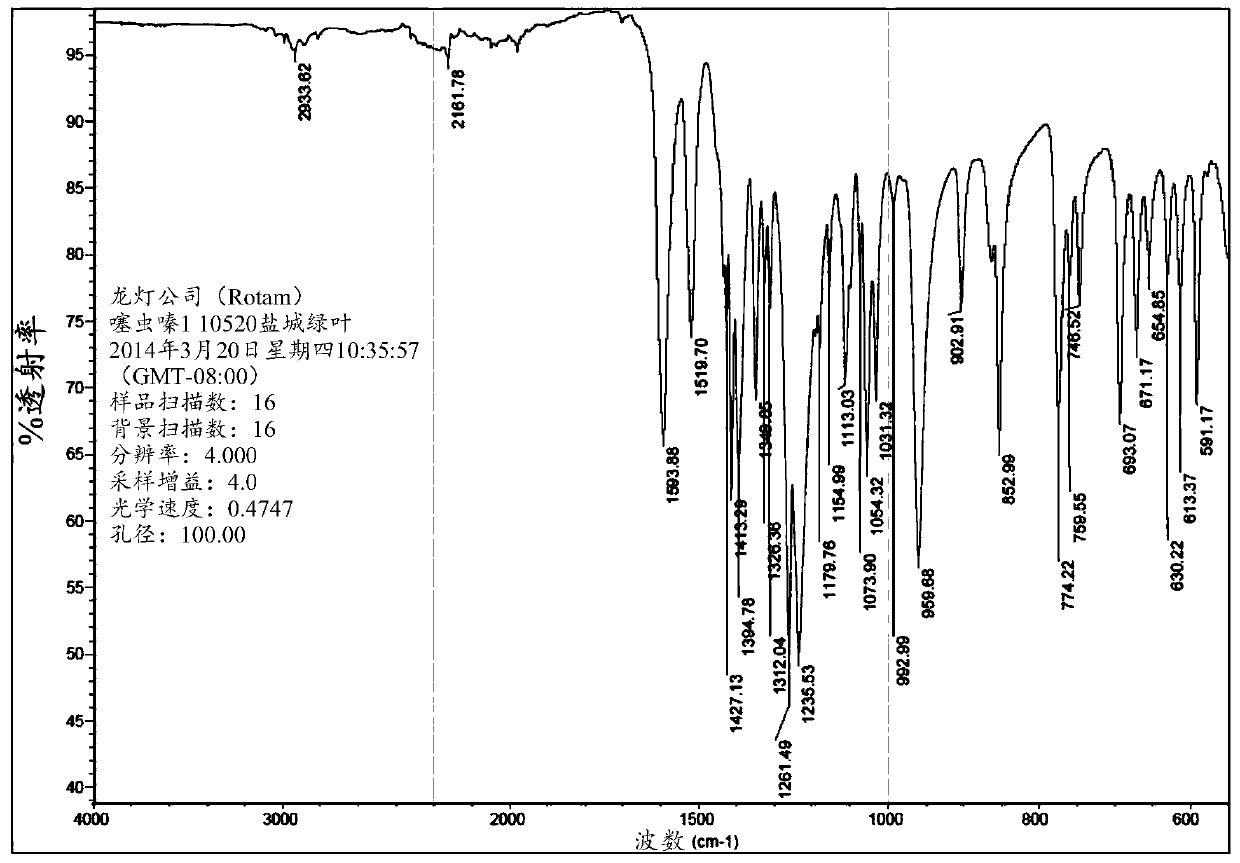
[0102] The IR spectrum of Form A thiamethoxam is listed in figure 2 middle. The IR spectra exhibited at 2933.62, 2161.78 and 1593.88cm -1 characteristic peaks at .
Embodiment 3
[0103] Example 3: Preparation of Crystalline Thiamethoxam Using Xylene
[0104] 2 g of thiamethoxam prepared as described in Example 1 was dissolved in 10 g of xylene while applying low heat on a hot plate. The resulting mixture was heated at reflux for 30 to 60 minutes and then cooled to room temperature. The resulting mixture was filtered to separate solids. After filtration, the solid was washed several times with xylene and dried under high vacuum to give technical grade pure thiamethoxam crystals (purity: 97%).
[0105] The crystals were characterized as Thiamethoxam Form A using infrared spectroscopy and X-ray diffraction as described in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com