Injectable double-drug-loading compound chitosan hydrogel and preparation method thereof
A technology of compounding chitosan water and dual drugs is applied in the field of medical biomaterials, which can solve the problem that the drug-loaded hydrogel cannot be applied in stages, and achieves the effects of good application prospects, resistance to inflammation, and no special requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
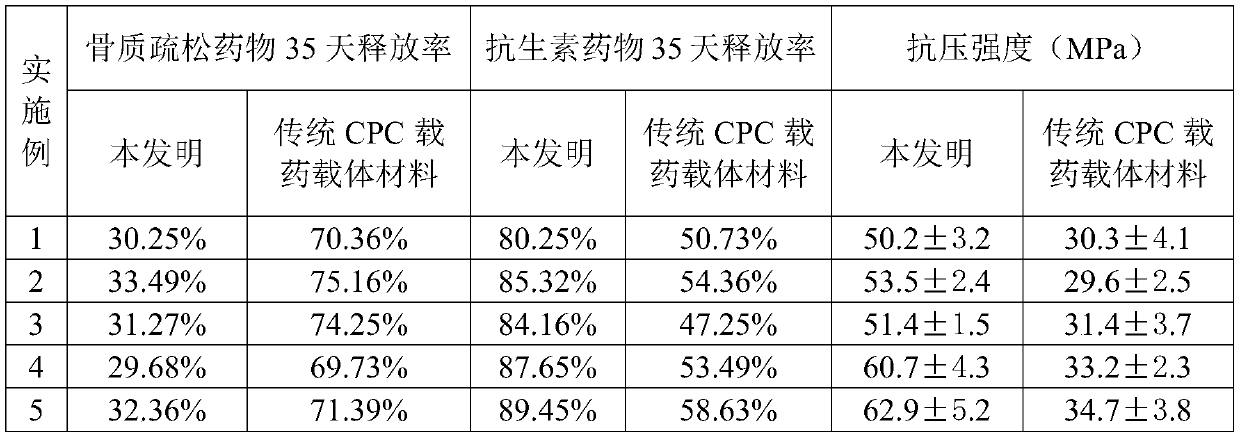
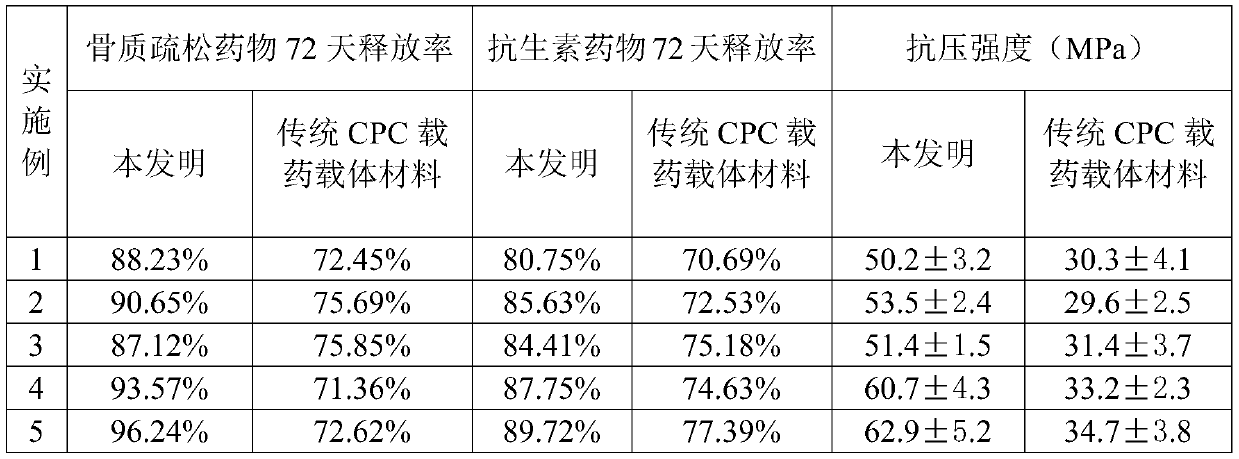
[0032] A kind of preparation method of injectable double-drug loaded composite chitosan hydrogel of the present invention comprises the following steps:
[0033] Step 1, preparing liposomes, fully dissolving lipids and osteoporosis drugs with a mass ratio of 0.5-1.5:0.01-0.5 in ether, then evaporating to remove ether in the solution, adding phosphate buffer to the solution, mixing Evenly, the drug-loaded liposome solution is obtained, and the drug-loaded liposome solution is dried and ground to obtain liposomes, and the lipid is phospholipid or sterol;
[0034] Step 2, taking an appropriate amount of chitosan and adding it into an acetic acid solution with a concentration of 0.525g / l to 3.15g / l, stirring evenly to obtain a light yellow transparent chitosan solution, the amount of chitosan in the chitosan solution The concentration is 2g / l~50g / l;
[0035] Step 3, Preparation of Hydrogel
[0036] Step 3.1, add liposomes into the chitosan solution, then add calcium nitrate, dis...
Embodiment 1
[0041] A kind of preparation method of injectable loaded double drug composite chitosan hydrogel is prepared, comprising the following steps:
[0042] Step 1, preparation of osteoporosis drug-loaded liposomes
[0043] Dissolve 1 g of phospholipids and 0.05 g of active vitamin D in ether in a container. After fully dissolving, remove the ether by rotary evaporation to form a liposome film on the surface of the container. Add 5 mL of phosphate buffer at pH=7 to the solution. The liposome membrane is fully shaken to hydrate and fall off to obtain a liposome solution, which is then dried and ground to obtain the osteoporosis drug-loaded liposome.
[0044] Step 2, prepare chitosan solution
[0045] Weigh 0.1g of chitosan and add it into 15mL of acetic acid solution, the concentration of the acetic acid solution is 0.525g / l, and fully dissolve it by magnetic stirring at 40°C to obtain a light yellow transparent liquid, namely chitosan solution;
[0046] Step 3, Preparation of Hydr...
Embodiment 2
[0052] A kind of preparation method of injectable loaded double drug composite chitosan hydrogel is prepared, comprising the following steps:
[0053] Step 1, preparation of osteoporosis drug-loaded liposomes
[0054] Dissolve 1.5g of phospholipids and 0.05g of alendronate sodium in ether in a container. After fully dissolving, remove the ether by rotary evaporation to form a liposome film on the surface of the container. Add 10mL of phosphate at pH=7 to the solution The buffer solution is fully shaken to hydrate and shed the liposome membrane to obtain a liposome solution, which is then dried and ground to obtain the osteoporosis drug-loaded liposome.
[0055] Step 2, prepare chitosan solution
[0056] Weigh 0.3g of chitosan and add it into 35mL of acetic acid solution, the concentration of the acetic acid solution is 1.5g / l, and fully dissolve it by magnetic stirring at 40°C to obtain a light yellow transparent liquid, namely chitosan solution;
[0057] Step 3, Preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com