Arsenic trioxide nanoparticles carrying transition metal complex and preparation method of arsenic trioxide nanoparticles
An arsenic trioxide, transition metal technology, applied in the fields of nanotechnology, nanotechnology, nanotechnology for materials and surface science, and can solve the problems of short maintenance time, low bioavailability, and low concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0025] The preparation of embodiment 1 arsenic trioxide nanoparticles
[0026] Accurately weigh 20mg PEG 5K -PLGA 20k Dissolve 2.5mg CO-520 in 1mL dichloromethane to form an organic phase; accurately weigh 50mg arsenic trioxide and dissolve it in sodium hydroxide, then adjust the pH with hydrochloric acid (pH=8) to prepare arsenic trioxide solution; take 50μL arsenic trioxide solution respectively Add water and 0.5mL organic phase, probe ultrasonic (over 2s, stop 2s) for 1 minute to obtain emulsion, mix the two emulsions and vortex for 5 minutes, then add to 5mL water probe ultrasonic (over 2s, stop 2s) for 2 minutes , followed by rotary evaporation at 40°C for 20 minutes to remove dichloromethane to obtain nanoparticles.
Embodiment 2
[0027] Example 2 Preparation of arsenic trioxide nanoparticles loaded with arsenic-nickel metal complex
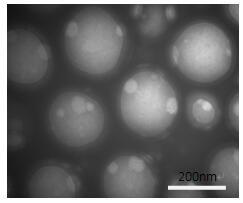
[0028] Accurately weigh 20mg PEG 5K -PLGA 20k Dissolve 2.5mg CO-520 in 1mL dichloromethane to form an organic phase; accurately weigh 50mg of arsenic trioxide and dissolve it in sodium hydroxide, then adjust the pH with hydrochloric acid (pH=8) to prepare arsenic trioxide solution, and accurately weigh 60mg of acetic acid Dissolve nickel in 1mL water to obtain nickel acetate solution; add 50μL arsenic trioxide solution and nickel acetate solution to 0.5mL organic phase respectively, probe ultrasonically (super 2s, stop 2s) for 1 minute to obtain emulsion, mix the two emulsions and vortex for 5 Minutes later, it was added to 5mL water and the probe was sonicated (super 2s, stop 2s) for 2 minutes, and then 40°C rotary evaporation for 20 minutes to remove dichloromethane to obtain nanoparticles. The electron microscope photo is shown in figure 1 .
Embodiment 3
[0029] Example 3 Preparation of arsenic trioxide nanoparticles loaded with arsenic-manganese metal complex
[0030] Accurately weigh 20mg PEG 5K -PLGA 20k Dissolve 2.5mg CO-520 in 1mL dichloromethane to form an organic phase; accurately weigh 50mg of arsenic trioxide and dissolve it in sodium hydroxide, then adjust the pH with hydrochloric acid (pH=8) to prepare arsenic trioxide solution, and accurately weigh 60mg of acetic acid Dissolve manganese in 1mL water to obtain manganese acetate solution; add 50μL of arsenic trioxide solution and manganese acetate solution to 0.5mL organic phase respectively, probe ultrasonically (super 2s, stop 2s) for 1 minute to obtain emulsion, mix the two emulsions and vortex for 5 Minutes later, it was added to 5mL of water and the probe was sonicated (super 2s, stop 2s) for 2 minutes, followed by rotary evaporation at 40°C for 20 minutes to remove dichloromethane to obtain nanoparticles.
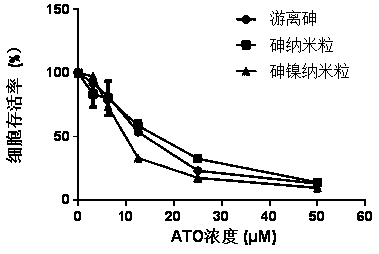
[0031] Determination of cell viability: K562 cells we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com