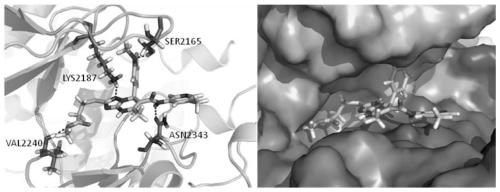
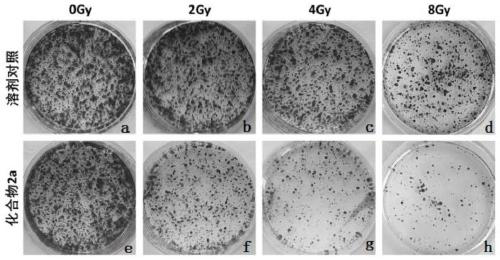
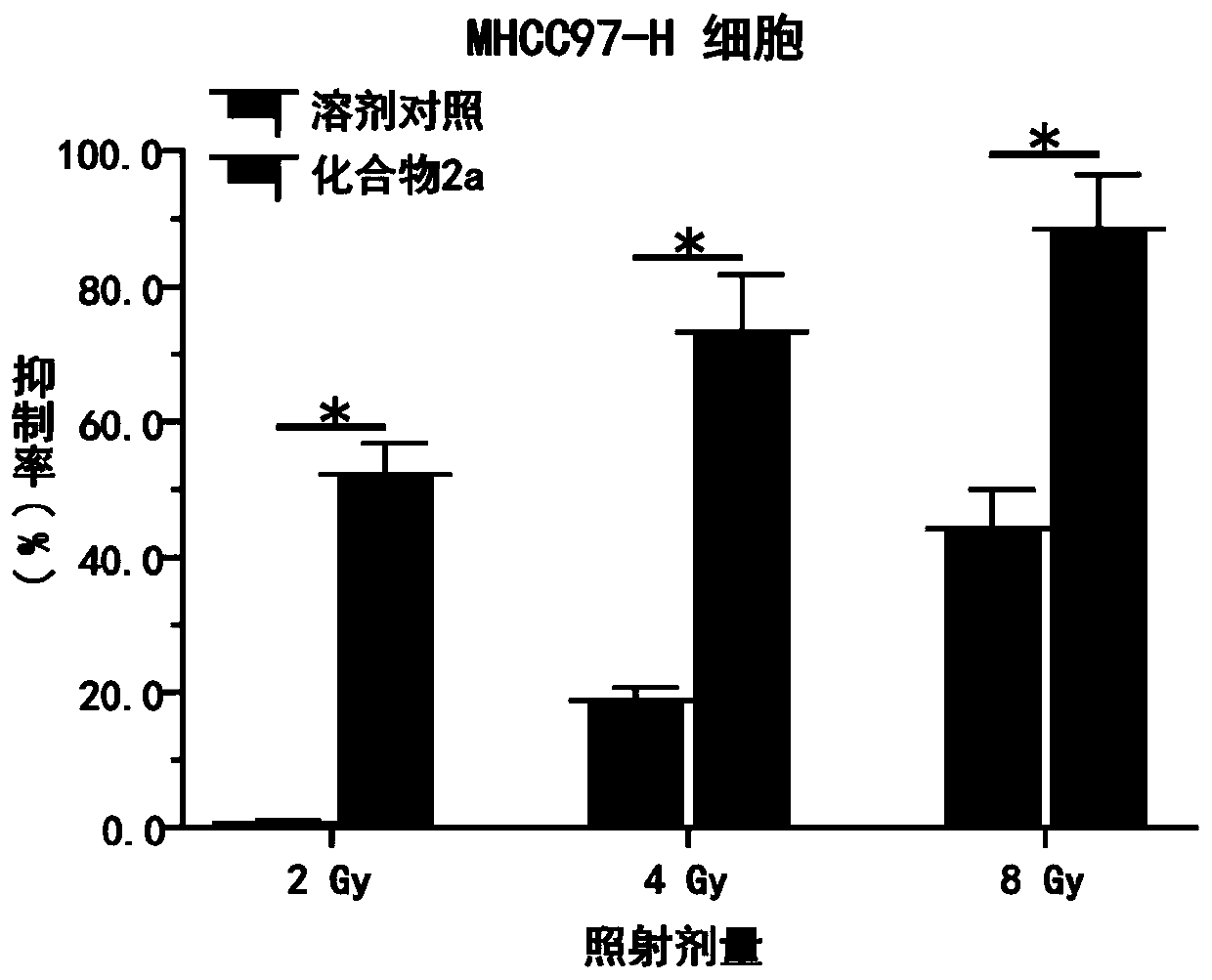
Triazolopyrimidine compound, preparation method and application thereof
A kind of technology of azolopyrimidine and compound, applied in the field of triazolopyrimidine compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] Methyl 3-((7-(3-methoxyphenyl)-5-methyl-6-((4-methylpyridin-3-yl)carbamoyl)-4,7-dihydro-[1 ,2,4]Synthesis of triazol[1,5-a]pyrimidin-2-yl)thio)propionate (2a)
[0077] Step 1: Preparation of methyl 3-((5-amino-1H-1,2,4-triazol-3-yl)thio)propionate (intermediate 1)
[0078] Methyl 3-bromopropionate (14.36g, 0.086mol) was dissolved in 40ml of absolute ethanol for later use. In a 500ml reaction vessel, NaOH (3.44g, 0.086mol) was dissolved in 200ml of purified water and stirred until completely dissolved. 3-Amino-5-thio-1,2,4-triazole (10.00 g, 0.086 mol) was then added and stirred. After 30 minutes, a mixture of methyl 3-bromopropionate and ethanol was added. React overnight at room temperature. After the reaction was complete, it was extracted with ethyl acetate, washed with saturated sodium chloride and washed with Na 2 SO 4 dry. Methyl 3-((5-amino-1H-1,2,4-triazol-3-yl)thio)propanoic acid (11.65 g, yield 67%) was obtained.
[0079] Step 2: Preparatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com