Desloratadine citrate disodium oral liquid preparation as well as preparation method and application thereof
A technology of desloratadine and loratadine is applied in the field of pharmaceutical preparations to achieve the effects of high quality taste, improved compliance and guaranteed safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
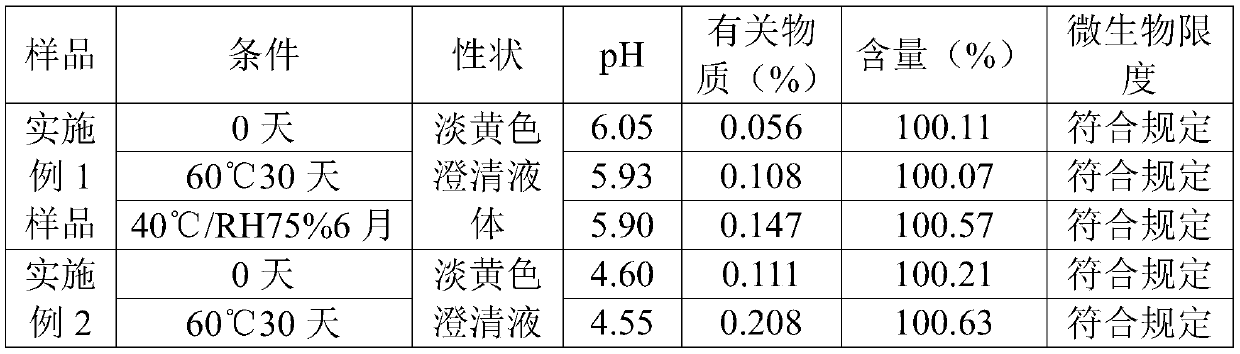
Examples
Embodiment 1
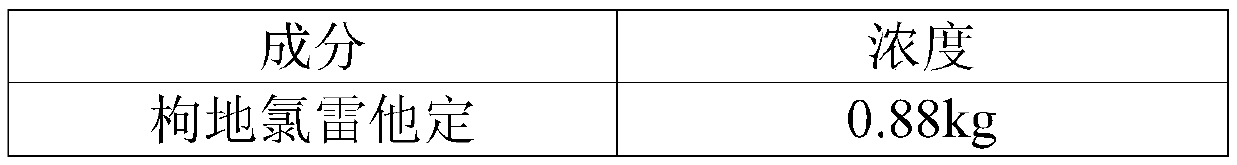
[0046] Embodiment 1 desloratadine citrate oral solution
[0047] Element concentration desloratadine citrate 0.88kg Ethylparaben 0.25kg sucrose 400kg Edetate Disodium 3kg sodium citrate 2kg apple flavor 1.5kg Add water to 1000L
[0048] The preparation method of this embodiment is:
[0049] (1) Desloratadine citrate, ethylparaben, sucrose, edetate disodium, sodium citrate, apple flavor are taken by weighing prescription quantity;
[0050] (2) Add the prescribed amount of ethylparaben and sucrose into an appropriate amount of purified water, stir and boil for about 20 minutes, and cool to room temperature;
[0051] (3) Add desloratadine citrate, disodium edetate, sodium citrate, and apple essence to the above solution, stir for about 30 minutes until dissolved, add purified water to 1000L, fill it, and obtain.
Embodiment 2
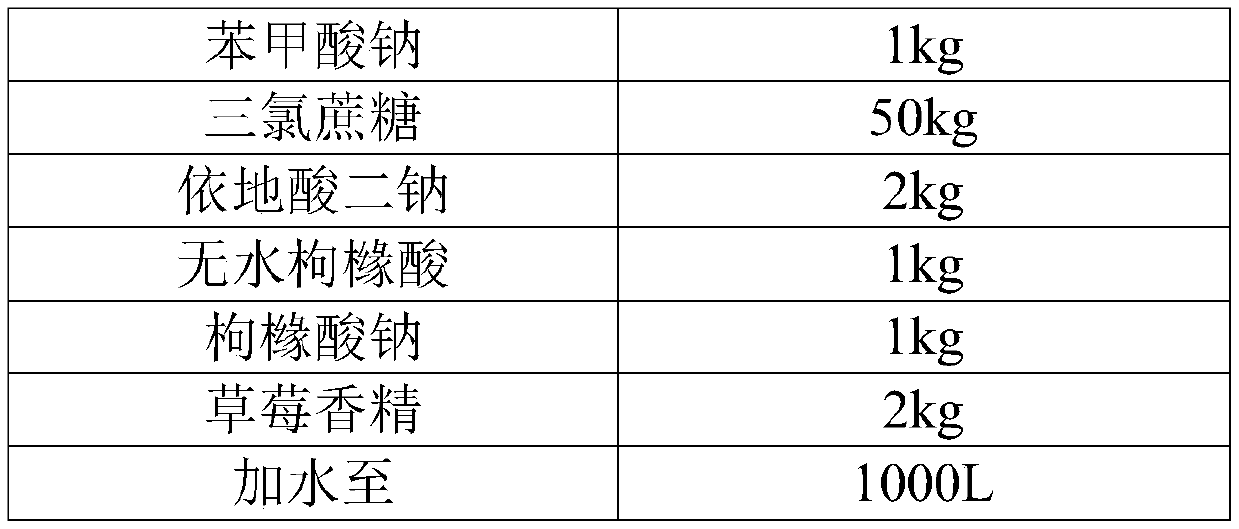
[0052] Embodiment 2 desloratadine citrate oral solution
[0053]
[0054]
[0055] The preparation method of this embodiment is:
[0056] (1) Desloratadine citrate, sodium benzoate, sucralose, edetate disodium, anhydrous citric acid, sodium citrate, and strawberry essence are weighed in prescription quantities;
[0057] (2) adding the sodium benzoate of prescription quantity into appropriate purified water, stirring and dissolving;
[0058] (3) Add desloratadine citrate, sucralose, edetate disodium, anhydrous citric acid, sodium citrate, strawberry essence to the above solution, stir for about 30 minutes until dissolved, add purified water to 1000L, filled and ready to serve.
Embodiment 3
[0059] Embodiment 3 desloratadine citrate oral solution
[0060] Element concentration desloratadine citrate 0.88kg Methylparaben 0.3kg Propylparaben 0.03kg Sorbitol 10kg Xylitol 15kg Sodium dihydrogen phosphate 0.3kg Disodium phosphate 4.0kg Edetate Disodium 1.5kg orange essence 1.0kg Amaranth 0.1kg Add water to 1000L
[0061] The preparation method of this embodiment is:
[0062] (1) Weigh desloratadine citrate, methylparaben, propylparaben, sorbitol, xylitol, sodium dihydrogen phosphate, disodium hydrogen phosphate, disodium edetate, orange essence in the prescribed amount , amaranth;
[0063] (2) Add the prescribed amount of methylparaben and propylparaben to an appropriate amount of purified water, stir and boil for 20 minutes to dissolve, and cool to room temperature;
[0064] (3) Add desloratadine citrate, sorbitol, xylitol, sodium dihydrogen phosphate, disodium hydrogen phospha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com